|
|
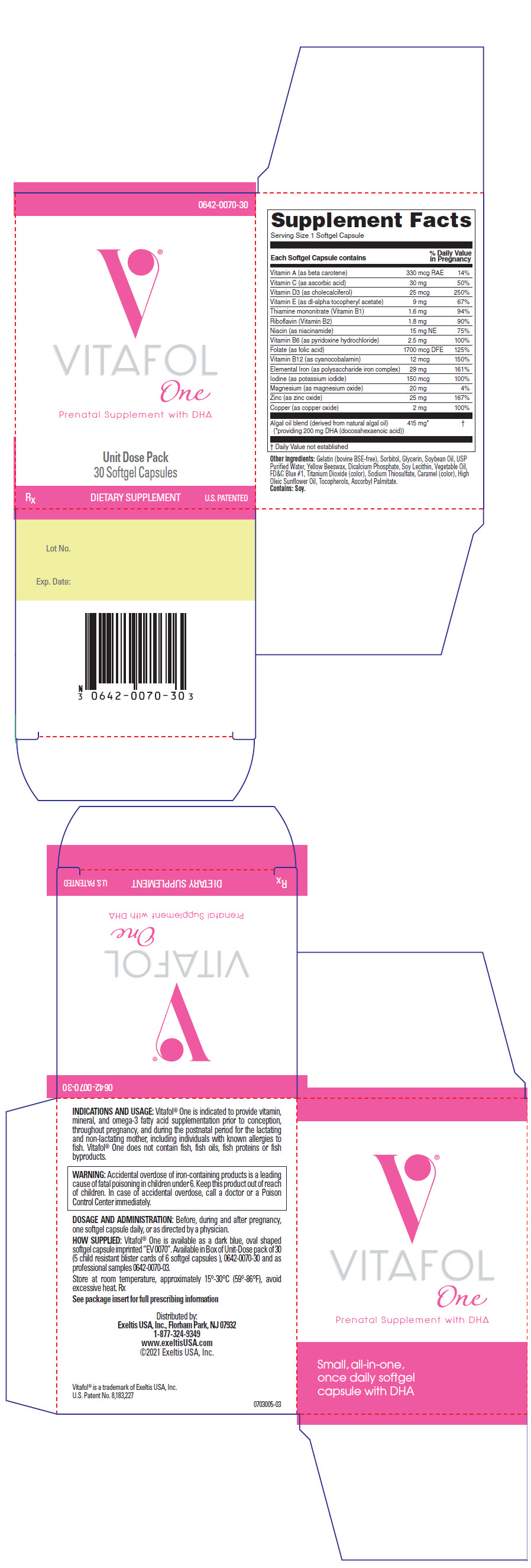
| COMPOSITION: | |
| Each Softgel Capsule Contains: | |
| VITAMINS AND MINERALS: | |
| Vitamin A (as Vitamin A palmitate) | 330 mcg RAE |
| Vitamin C (as ascorbic acid) | 30 mg |
| Vitamin D3 (as cholecalciferol) | 25 mcg |
| Vitamin E (as dl-alpha tocopheryl acetate) | 9 mg |
| Thiamine mononitrate (Vitamin B1) | 1.6 mg |
| Riboflavin (Vitamin B2) | 1.8 mg |
| Niacin (as niacinamide) | 15 mg NE |
| Vitamin B6 (as pyridoxine hydrochloride) | 2.5 mg |
| Folate (as folic acid) | 1700 mcg DFE |
| Vitamin B12 (as cyanocobalamin) | 12 mcg |
| Elemental Iron (as polysaccharide iron complex) | 29 mg |
| Iodine (as potassium iodide) | 150 mcg |
| Magnesium (as magnesium oxide) | 20 mg |
| Zinc (as zinc oxide) | 25 mg |
| Copper (as copper oxide) | 2 mg |
| Algal oil blend (derived from natural algal oil) | 415 mg* |
Other Ingredients:
Gelatin (Bovine BSE-free), Sorbitol, Glycerin, Soybean Oil, USP Purified Water, Yellow Beeswax, Dicalcium Phosphate, Soy Lecithin, Vegetable Oil, FD&C Blue #1, Titanium Dioxide (color), Sodium Thiosulfate, Caramel (color), High Oleic Sunflower Oil, Tocopherols, Ascorbyl Palmitate. Contains: Soy.
INDICATIONS AND USAGE
Vitafol-One is indicated to provide vitamin, mineral, and omega-3 fatty acid supplementation prior to conception, throughout pregnancy, and during the postnatal period for the lactating and non-lactating mother, including individuals with known allergies to fish. Vitafol-One does not contain fish, fish oils, fish proteins or fish byproducts.
CONTRAINDICATIONS
Vitafol-One is contraindicated in patients with hypersensitivity to any of its components or color additives.
Folic acid is contraindicated in patients with untreated and uncomplicated pernicious anemia, and in those with anaphylactic sensitivity to folic acid.
Iron therapy is contraindicated in patients with hemochromatosis and patients with iron storage disease or the potential for iron storage disease due to chronic hemolytic anemia (e.g., inherited anomalies of hemoglobin structure or synthesis and/or red cell enzyme deficiencies, etc.), pyridoxine responsive anemia, or cirrhosis of the liver.
Cyanocobalamin is contraindicated in patients with sensitivity to cobalt or to cyanocobalamin (vitamin B-12).
WARNING
Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor or a Poison Control Center immediately.
WARNINGS/PRECAUTIONS
Vitafol-One should be used with caution in patients with known sensitivity or allergy to soy.
Vitamin D supplementation should be used with caution in those with hypercalcemia or conditions that may lead to hypercalcemia such as hyperparathyroidism and those who form calcium-containing kidney stones. High doses of vitamin D can lead to elevated levels of calcium that reside in the blood and soft tissues. Bone pain, high blood pressure, formation of kidney stones, renal failure, and increased risk of heart disease can occur.
Iodine should be used with caution in patients with an overactive thyroid.
Prolonged use of iron salts may produce iron storage disease. Folic acid, especially in doses above 0.1 mg daily, may obscure pernicious anemia, in that hematologic remission may occur while neurological manifestations remain progressive.
The use of folic acid doses above 1 mg daily may precipitate or exacerbate the neurological damage of vitamin B12 deficiency.
Consumption of more than 3 grams of omega-3 fatty acids per day from all sources may lead to excessive bleeding. Supplemental intake of omega-3 fatty acids such as DHA exceeding 2 grams per day is not recommended.
Avoid Overdosage. Keep out of the reach of children.
Drug Interactions
Medications for an overactive thyroid (anti-thyroid drugs) used in conjunction with iodine supplementation may lead to hypothyroidism.
Medications for hypertension used in conjunction with iodine supplementation may increase potassium.
High doses of folic acid may result in decreased serum levels of the anticonvulsant drugs; carbamazepine, fosphenytoin, phenytoin, phenobarbitol, valproic acid. Folic acid may decrease a patient's response to methotrexate.
Vitamin D supplementation should not be given with large amounts of calcium in those with hypercalcemia or conditions that may lead to hypercalcemia such as hyperparathyroidism and those who form calcium-containing kidney stones.
Zinc can inhibit the absorption of certain antibiotics; taken at least 2 hours apart to minimize interactions.
Consult appropriate references for additional specific vitamin drug interactions.
ADVERSE REACTIONS
Adverse reactions have been reported with specific vitamins and minerals, but generally at doses substantially higher than those in Vitafol-One. However, allergic and idiosyncratic reactions are possible at any dose. Reported adverse events include skin ailments, gastrointestinal complaints, glucose abnormalities, and visual problems.
DOSAGE AND ADMINISTRATION
Before, during and after pregnancy, one softgel capsule daily, or as directed by a physician.
HOW SUPPLIED
Vitafol-One is available as a dark blue, oval shaped softgel capsule imprinted "EV0070". Available in Box of Unit-Dose pack of 30 (5 child resistant blister cards of 6 softgel capsules), (0642-0070-30) and as professional samples (0642-0070-03).