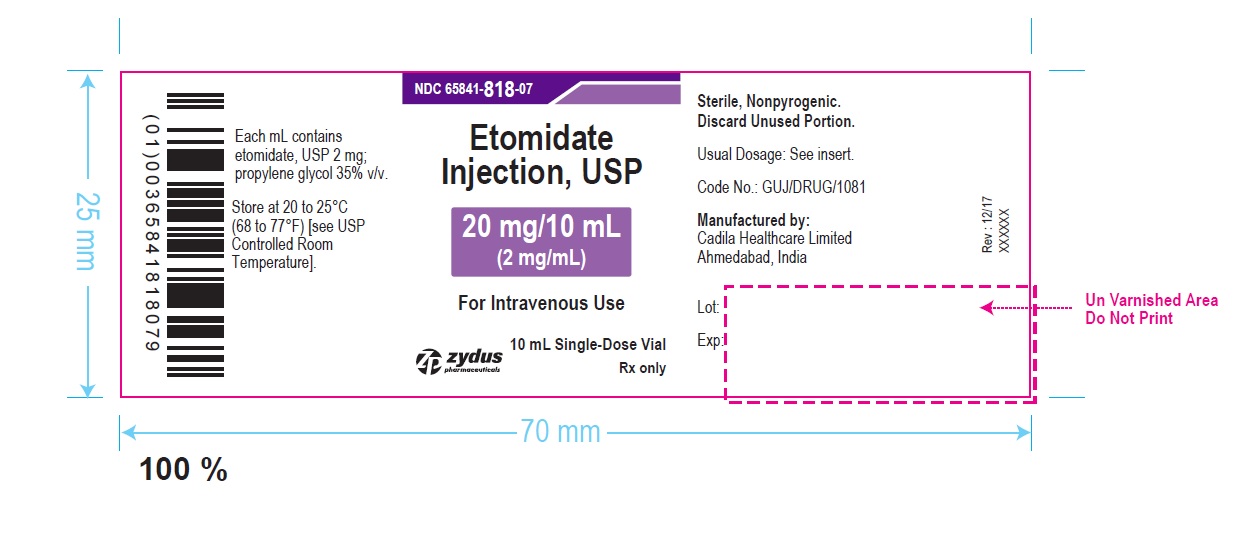
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Zydus Pharmaceuticals
NDC 65841-818-07
Etomidate Injection, USP
20 mg/10 mL
(2 mg/mL)
For Intravenous Use
10 mL, Single-Dose Vial
Rx Only
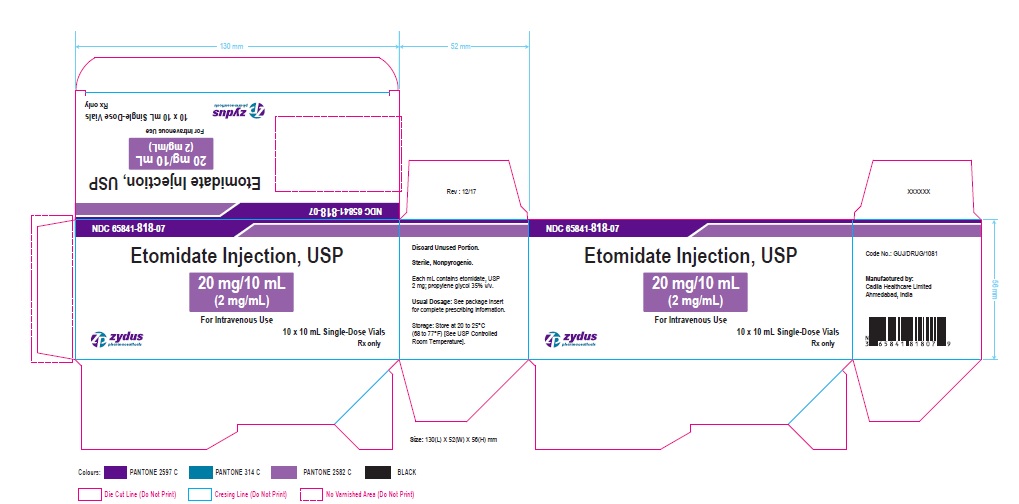
Zydus Pharmaceuticals
NDC 65841-818-07
Etomidate injection, USP
20 mg/10 mL
(2 mg/mL)
For Intravenous Use
10 x 10 mL, Single-Dose Vials
Rx Only
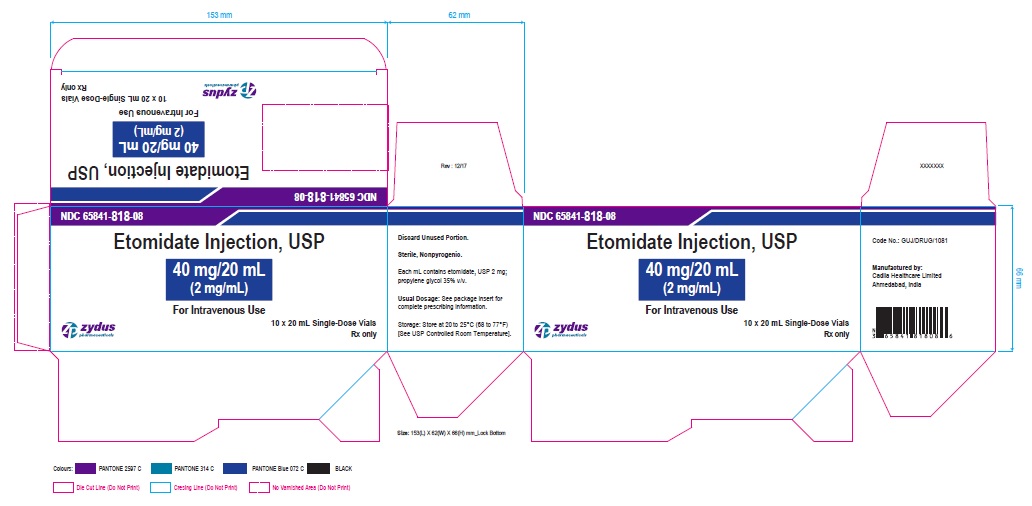
Zydus Pharmaceuticals
NDC 65841-818-08
Etomidate injection, USP
40 mg/20 mL
(2 mg/mL)
For Intravenous Use
20 mL, Single-Dose Vial
Rx Only
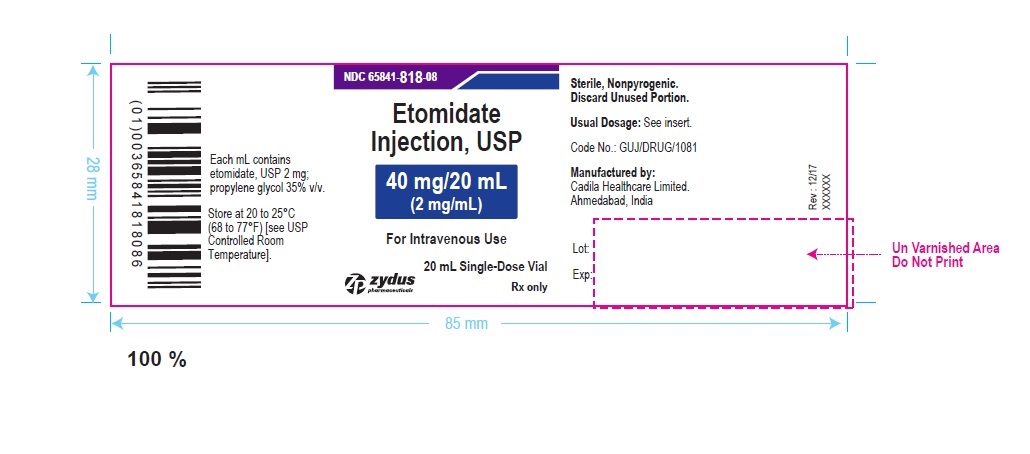
Zydus Pharmaceuticals
NDC 65841-818-08
Etomidate injection, USP
40 mg/20 mL
(2 mg/mL)
For Intravenous Use
10 x 20 mL, Single-Dose Vials
Rx Only