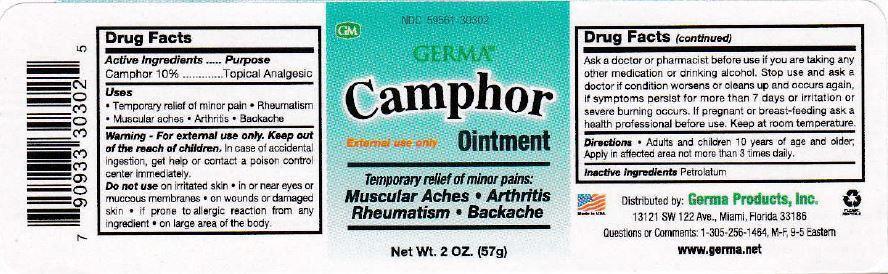
Camphor Ointment
External Use Only
Temporary relief of minor pains:
Muscular Aches | Arthritis
Rheumatism | Backache
Directions - Adults and children 10 years of age and older: apply in affected area not more than 3 times daily.
Warning - For external use only.
In case of accidental ingestion, get help or contact a poison control center immediately.
Do not use
- on irritated skin
- in or near eyes or mucous membranes
- on wounds or damaged skin
- if prone to allergic reaction from any ingredient
- on large area of the body
Ask a doctor or pharmacist before use if you are taking any other medication or drinking alcohol. Stop use and ask a doctor if condition worsens or clears up and occurs again, if symptoms persist for more than 7 days or irritation or severe burning occurs. If pregnant or breast-feeding ask a health professional before use. Keep at room temperature.