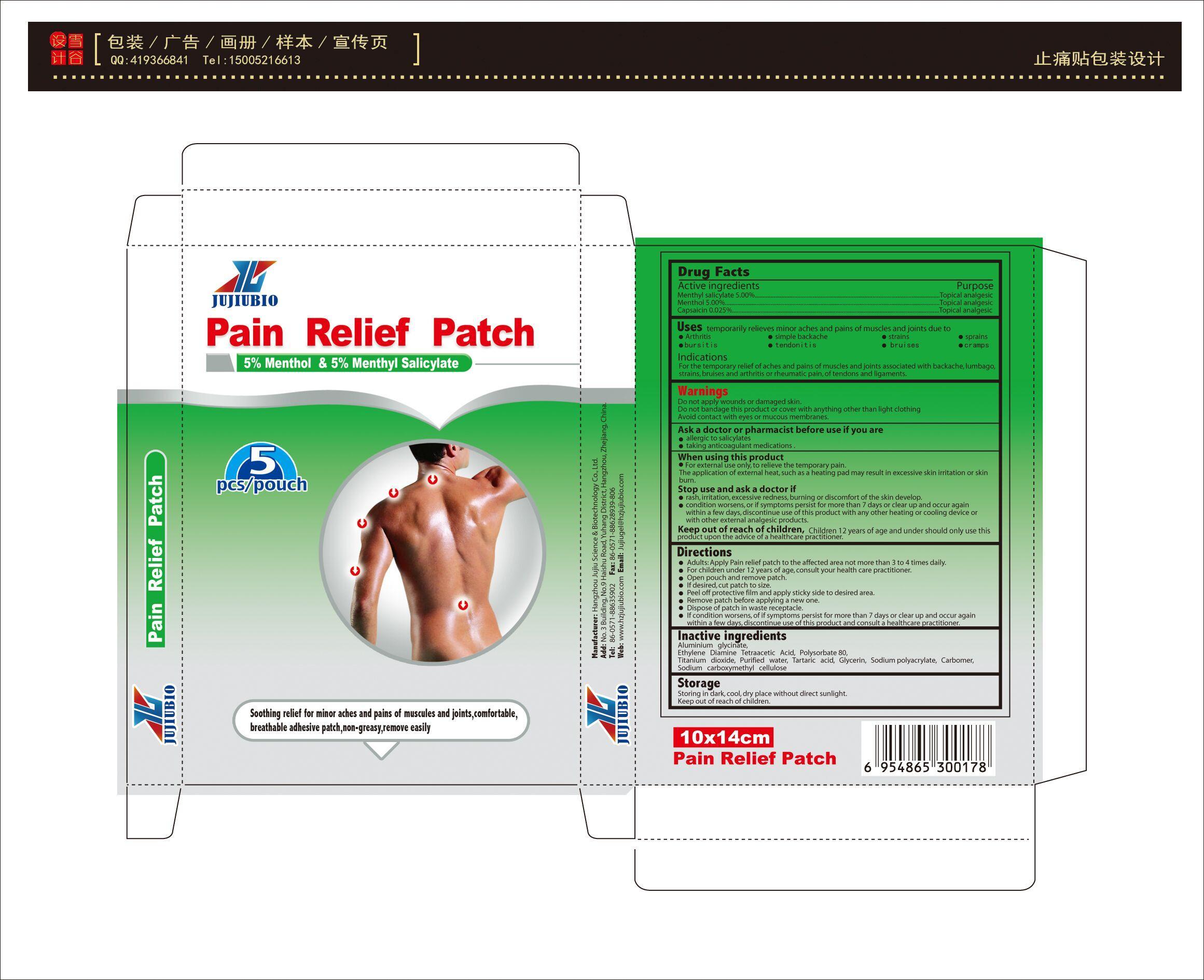
Warnning
Do not apply wounds or damaged skin.
Do not bandage this product or cover with anything other than light clothing
Avoid contact with eyes or mucous membranes.
Ask a doctor or pharmacist before use if you are
allergic to salicylates
taking anticoagulant medications .
When using this product
For external use only, to relieve the temporary pain.
The application of external heat, such as a heating pad may result in excessive skin irritation or skin burn.
Stop use and ask a doctor if
rash, irritation, excessive redness, burning or discomfort of the skin develop.
condition worsens, or if symptoms persist for more than 7 days or clear up and occur again within a few days, discontinue use of this product with any other heating or cooling device or with other external analgesic products.
Directions
Adults: Apply Pain relief patch to the affected area not more than 3 to 4 times daily.
For children under 12 years of age, consult your health care practitioner.
Open pouch and remove patch.
If desired, cut patch to size.
Peel off protective film and apply sticky side to desired area.
Remove patch before applying a new one.
Dispose of patch in waste receptacle.
If condition worsens, of if symptoms persist for more than 7 days or clear up and occur again within a few days, discontinue use of this product and consult a healthcare practitioner.