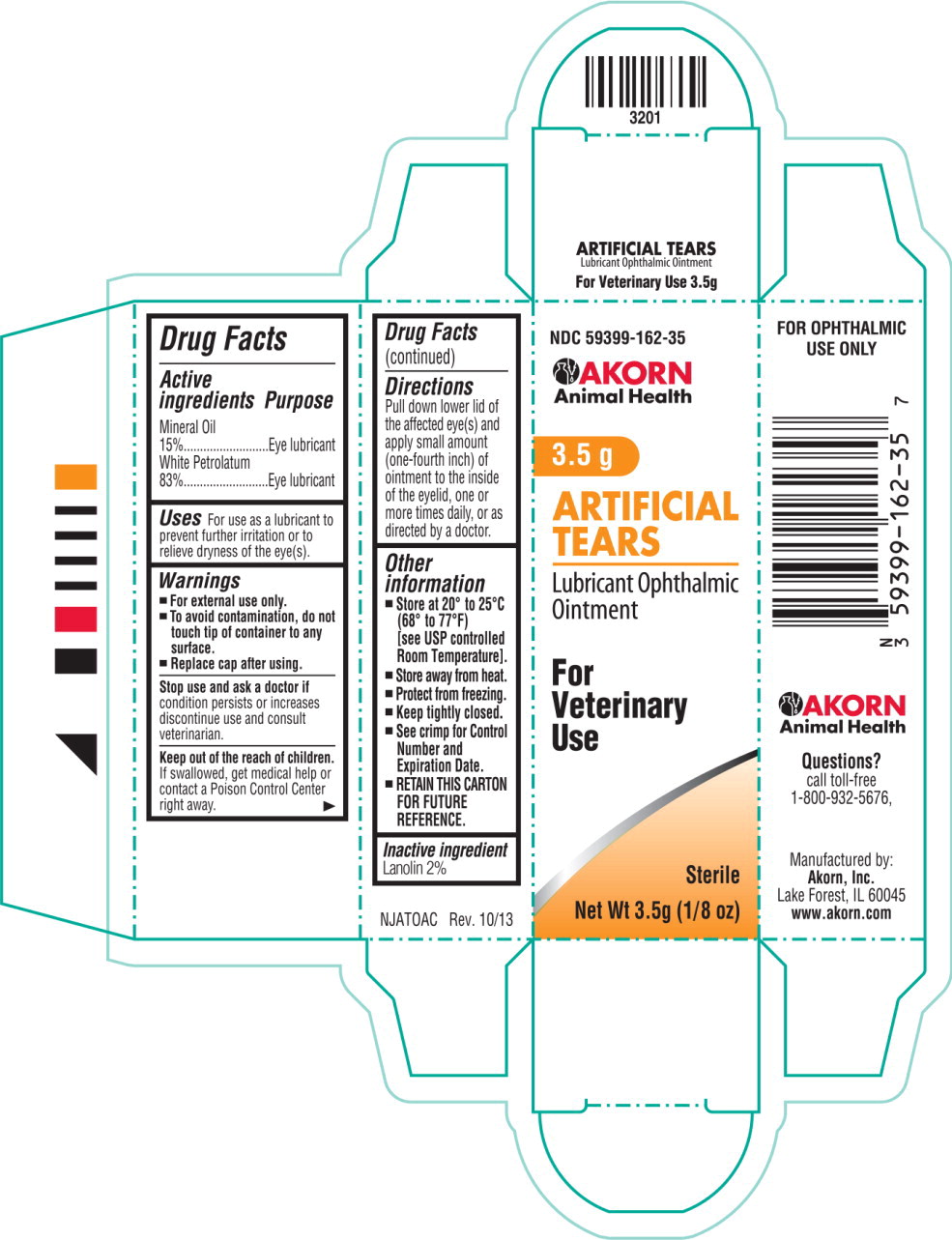
Active ingredients
Mineral Oil 15%
White Petrolatum 83%
Purpose
Eye lubricant
Eye lubricant
Uses
For use as a lubricant to prevent further irritation or to relieve dryness of the eye(s).
Warnings
-
For external use only.
-
To avoid contamination, do not touch tip of container to any surface.
-
Replace cap after using.
Stop use and ask a doctor if condition persists or increases discontinue use and consult veterinarian.
Keep out of the reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Pull down lower lid of the affected eye(s) and apply small amount (one-fourth inch) of ointment to the inside of the eyelid, one or more times daily, or as directed by a doctor.
Other information
-
Store at 20°
to 25°C (68°
to 77°F)
[see USP controlled Room Temperature].
-
Store away from heat.
-
Protect from freezing.
-
Keep tightly closed.
-
See crimp for Control Number and Expiration Date.
-
RETAIN THIS CARTON FOR FUTURE REFERENCE.
Inactive ingredient
Lanolin 2%
Principal Display Panel Text for Container Label:
NDC 59399-162-35 Akorn Animal Health Logo
3.5 g
ARTIFICIAL TEARS
Lubricant Ophthalmic Ointment FOR OPHTHALMIC USE ONLY
For Veterinary Use Net Wt 3.5g (1/8 oz) Sterile
Principal Display Panel Text for Carton Label:
NDC 59399-162-35
Akorn Animal Health Logo
3.5 g
ARTIFICIAL
TEARS
Lubricant Ophthalmic
Ointment
For
Veterinary
Use
Sterile
Net Wt 3.5g (1/8 oz)