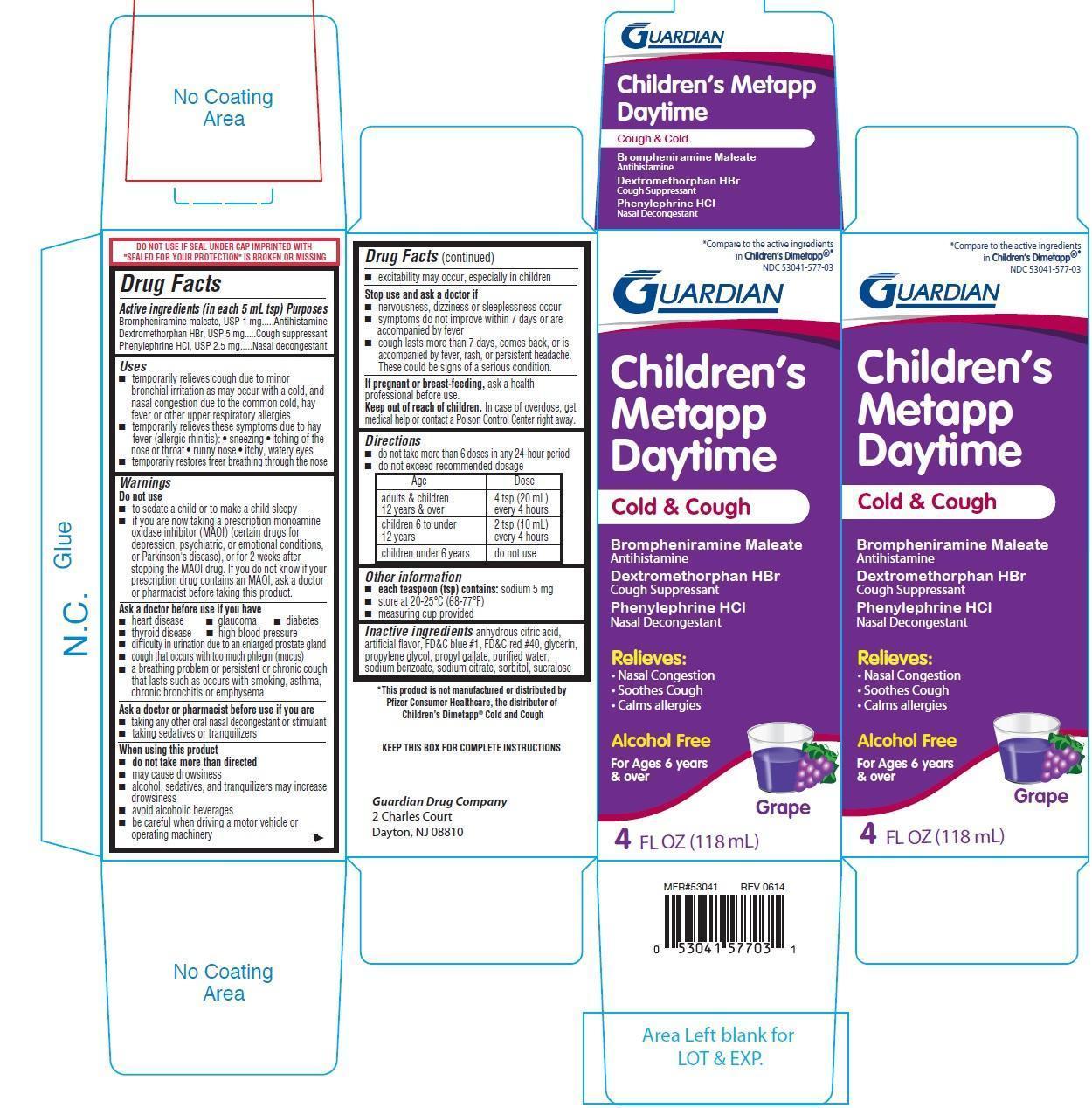
Active ingredients (in each 5 mL tsp)
Brompheniramine maleate 1 mg
Dextromethorphan HBr 5 mg
Phenylephrine HCl 2.5 mg
Uses
- temporarily relieves cough due to minor bronchial irritation as may occur with a cold, and nasal congestion due to the common cold, hay fever or other upper respiratory allergies
- temporarily relieves these symptoms due to hay fever (allergic rhinitis):
- sneezing
- itching of the nose or throat
- runny nose
- itchy, watery eyes
- temporarily restores freer breathing through the nose
Warnings
Do not use
- to sedate a child or to make a child sleepy
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI)(certain drugs for depression, psychiatric, or emotional conditions, or parkinsons disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- heart disease
- glaucoma
- diabetes
- thyroid disease
- high blood pressure
- difficulty in urination due to an enlarged prostate gland
- cough that occurs with too much phlegm (mucus)
- a breathing problem or persistent or chronic cough that lasts such as occurs with smoking asthma, chronic bronchitis or emphysema
Ask a doctor or pharmacist before use if you are
- taking any other oral nasal decongestant or stimulant
- taking sedatives or tranquilizers
when using this product
- do not take more than directed
- may cause drowsiness
- alcohol, sedatives, and tranquilizers may increase drowsiness
- avoid alcohol beverages
- be careful when driving a motor vehicle or operating mahcinery
- excitability may occur, especially in children
Directions
- do not take more than 6 doses in any 24-hour period
- do not exceed recommended dosage
| Age | Dose |
| adults and children 12 years and over | 4 tsp (20 mL) every 4 hours |
| children 6 to under 12 years | 2 tsp (10 mL) every 4 hours |
| children under 6 years | do not use |
Other information
- each teaspoon (tsp) contains: sodium 5 mg
- store at 20-25oC (68-77oF)
- measuring cup provided
Inactive ingredients
anhydrous citric acid, artificial flavor, FD&C BLUE #1, FD&C red 40, glycerin, propylene glycol, propyl gallate, purified water, sodium benzoate, sodium citrate, sorbitol, sucralose
PDP
Compare to the active ingredients in Childrens Dimetapp
Childrens Metapp Daytime
Cold & Cough
Brompheniramine Maleate
Antihistamine
Dextromethorphan HBr
Cough Suppressant
Phenylephrine HCl
Nasal Decongestant
Relieves:
- Nassal Congestion
- Soothes Cough
- Calms allergies
Alcohol Free
For ages 6 years and over
Grape
4 FL OZ (118 mL)