CVS Povidone-Iodine
Drug Facts
Active Ingredient
Povidone-Iodine 10% (1% Available iodine)
Purpose
First Aid Antiseptic
Uses
First aid to help prevent infection in in minor: cuts, scrapes and burns.
Warnings
For external use only
Do not use
in the eyes. Over large areas of the body.
Ask a doctor before use
in case of deep or puncture wounds, animal bites, or serious burns.
Stop use and consult a doctor if:
The condition persists or gets worse. Need to use this product for more than 1 week.
Keep out of reach of children.
- If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Clean the affected area. Apply a small amount to the affected area 1 to 3 times daily. May be covered with a sterile bandage. If bandaged, let dry first.
Inactive Ingredient
Citric acid, Dibasic sodium Phosphate, Glycerin, Nonoxynol-9, Purified water, sodium hydroxide.
PRINCIPAL DISPLAY PANEL
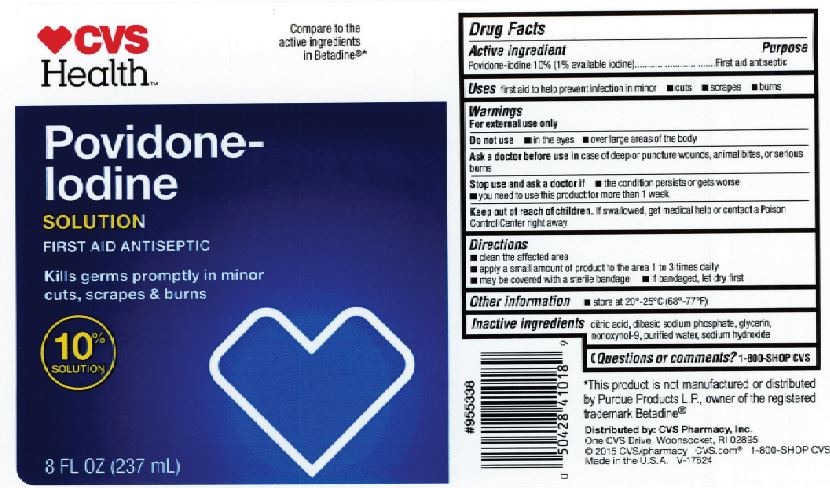 CVS Health
CVS Health
Povidone-
Iodine
8 FL OZ (237 mL)
