Uses
- •
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
- •
- temporarily relieves nasal congestion due to:
- •
- common cold
- •
- hay fever
- •
- upper respiratory allergies
- •
- temporarily restores freer breathing through the nose
- •
- promotes nasal and/or sinus drainage
- •
- temporarily relieves sinus congestion and pressure
Warnings
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- •
- heart disease
- •
- high blood pressure
- •
- thyroid disease
- •
- diabetes
- •
- trouble urinating due to an enlarged prostate gland
- •
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- •
- cough accompanied by too much phlegm (mucus)
Directions
- •
- do not crush, chew, or break tablet
- •
- take with a full glass of water
- •
- this product can be administered without regard for timing of meals
- •
- adults and children 12 years and older: 2 tablets every 12 hours; not more than 4 tablets in 24 hours
- •
- children under 12 years of age: do not use
Other information
- •
- Tamper evident: Do not use if carton is open or if printed seal on blister is broken or missing.
- •
- store between 20-25°C (68-77°F)
Inactive ingredients
carbomer homopolymer type B; hypromellose, USP; magnesium stearate, NF; microcrystalline cellulose, NF; sodium starch glycolate, NF
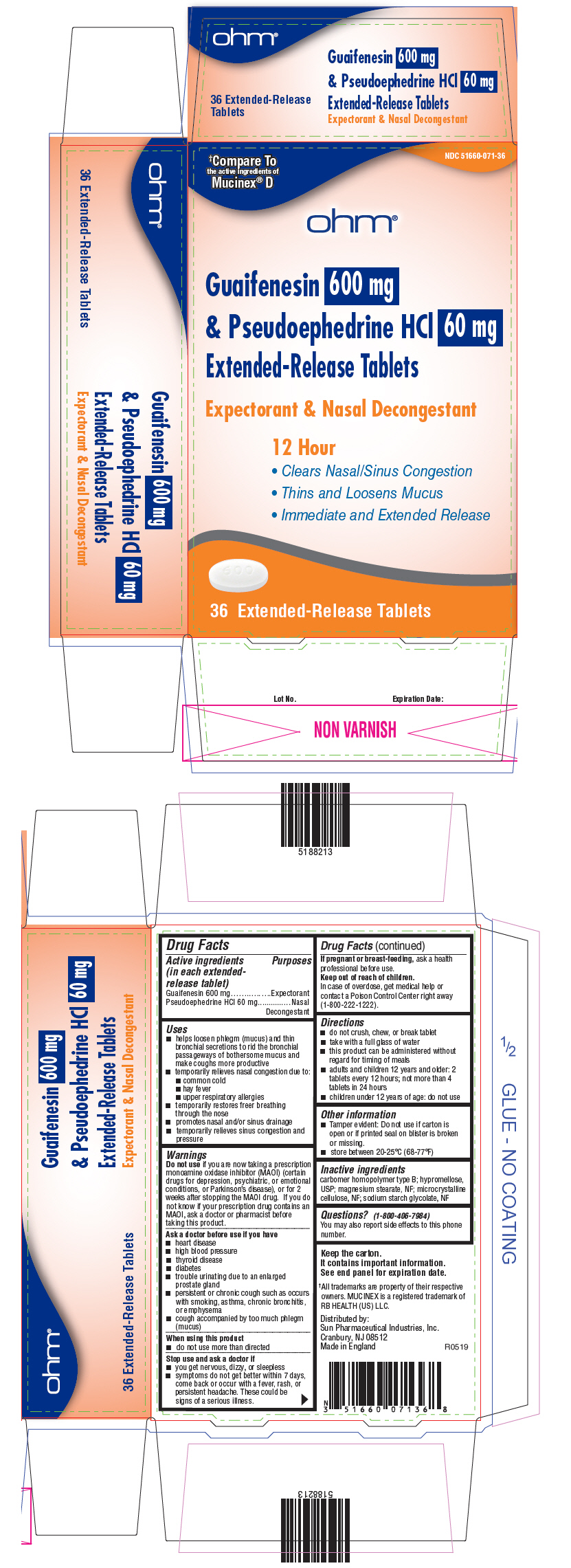
PRINCIPAL DISPLAY PANEL - 600 mg/60 mg Tablet Blister Pack Carton
†Compare To
the active ingredients of
Mucinex® D
NDC 51660-071-36
ohm®
Guaifenesin 600 mg
& Pseudoephedrine HCl 60 mg
Extended-Release Tablets
Expectorant & Nasal Decongestant
12 Hour
- Clears Nasal/Sinus Congestion
- Thins and Loosens Mucus
- Immediate and Extended Release
36 Extended-Release Tablets