PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 65841-052-01 in bottle of 100 tablets
Warfarin Sodium Tablets USP, 1 mg
Rx only
100 tablets

NDC 65841-053-01 in bottle of 100 tablets
Warfarin Sodium Tablets USP, 2 mg
Rx only
100 tablets

NDC 65841-064-01 in bottle of 100 tablets
Warfarin Sodium Tablets USP, 2.5 mg
Rx only
100 tablets

NDC 65841-054-01 in bottle of 100 tablets
Warfarin Sodium Tablets USP, 3 mg
Rx only
100 tablets

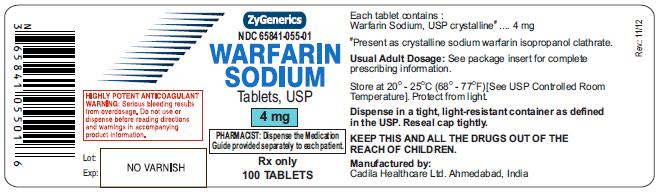
NDC 65841-055-01 in bottle of 100 tablets
Warfarin Sodium Tablets USP, 4 mg
Rx only
100 tablets
NDC 65841-056-01 in bottle of 100 tablets
Warfarin Sodium Tablets USP, 5 mg
Rx only
100 tablets

NDC 65841-057-01 in bottle of 100 tablets
Warfarin Sodium Tablets USP, 6 mg
Rx only
100 tablets

NDC 65841-058-01 in bottle of 100 tablets
Warfarin Sodium Tablets USP, 7.5 mg
Rx only
100 tablets

NDC 65841-059-01 in bottle of 100 tablets
Warfarin Sodium Tablets USP, 10 mg
Rx only
100 tablets
