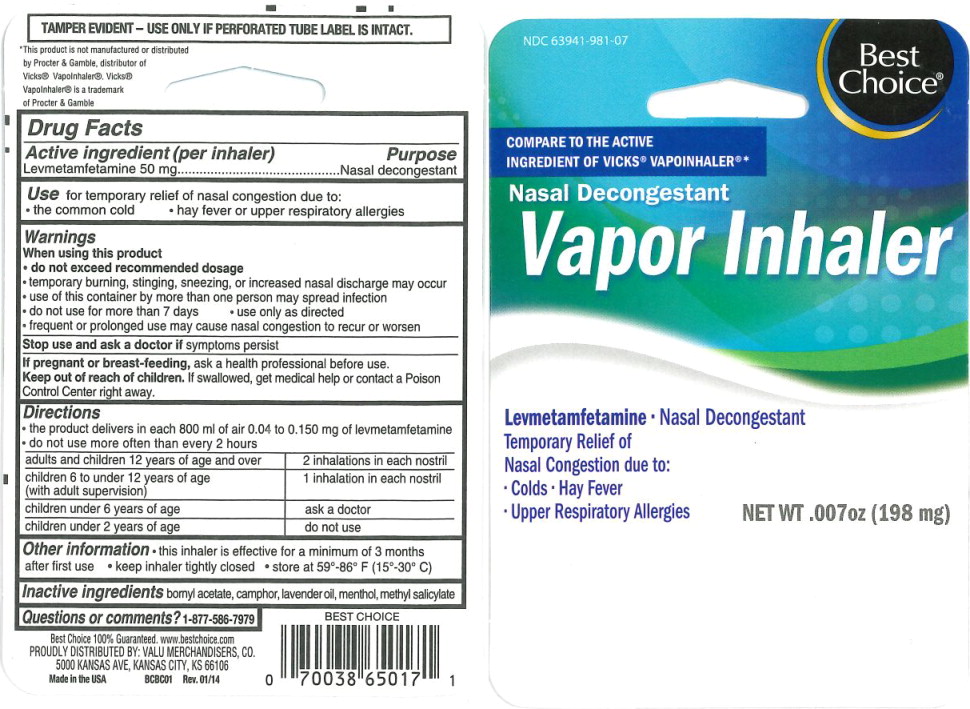
Use:
for temporary relief of nasal congestion due to:
- the common cold
- hay fever or upper respiratory allergies
Warnings
When using this product
- do not exceed recommended dosage
- temporary burning, stinging, sneezing, or increased nasal Squareharge may occur
- use of this container by more than one person may spread infection
Directions
- the product delivers in each 800 mL of air 0.04 to 0.150 mg of levmetamfetamine
- do not use more often than every 2 hours
| adults and children 12 years of age and over | 2 inhalations in each nostril |
| children 6 to under 12 years of age (with adult supervision) | 1 inhalation in each nostril |
| children under 6 years of age | ask a doctor |
| children under 2 years of age | do not use |
Other information
- this inhaler is effective for a minimum of 3 months after first use
- keep inhaler tightly closed
- store at 59°-86° F (15°-30° C)
Principal Display Panel - 15 mg Blister Pack Label
NDC 63941-981-07
Best
Choice®
COMPARE TO THE ACTIVE
INGREDIENT OF VICKS® VAPOINHALER®*
Nasal Decongestant
Vapor Inhaler
Levmetamfetamine
- Nasal Decongestant
Temporary Relief of
Nasal Congestion due to:
- Colds
- Hay Fever
- Upper Respiratory Allergies
NET WT .007 oz (198 mg)