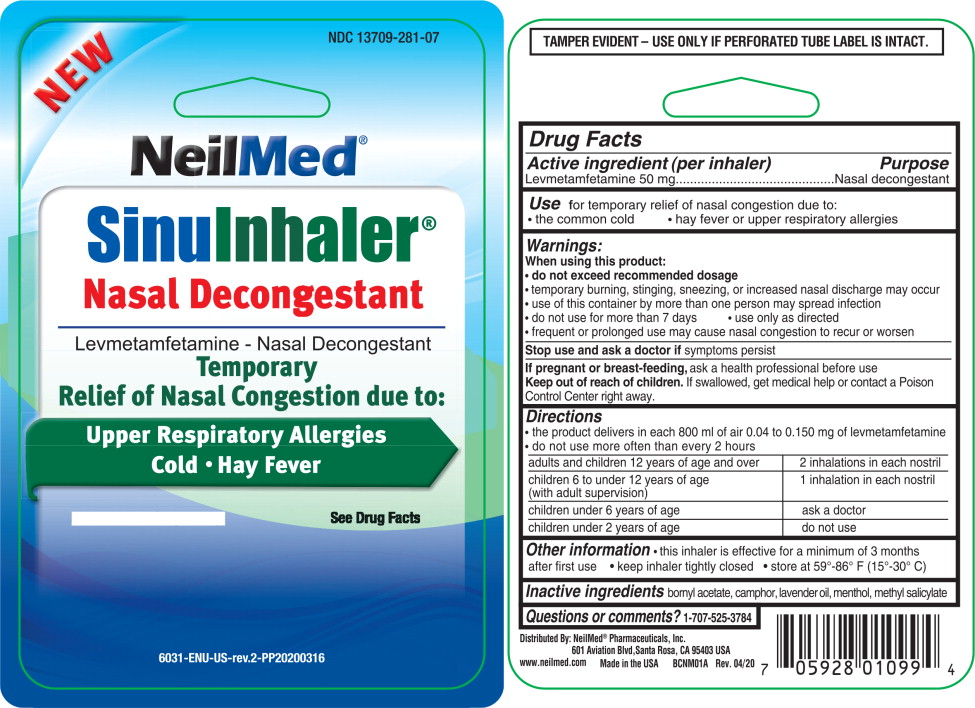
Use for temporary relief of nasal congestion due to:
- the common cold
- hay fever or upper respiratory allergies
When using this product
- do not exceed recommended dosage
- temporary burning, stinging, sneezing, or increased nasal discharge may occur
- use of this container by more than one person may spread infection
- do not use for more than 7 days
- Use only as directed
- frequent or prolonged use may cause nasal congestion to recur or worsen
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- the product delivers in each 800 ml of air 0.04 to 0.150 mg of levmetamfetamine
- do not use more often than every 2 hours
| adults and children 12 years of age and over | 2 inhalations in each nostril |
|
children 6 to under 12 years of age (with adult supervision) | 1 inhalation in each nostril |
| children under 6 years of age | ask a doctor |
| children under 2 years of age | do not use |
Other Information
- this inhaler is effective for a minimum of 3 months after first use
- keep inhaler tightly closed
- store at 59o-86oF (15o- 30oC)
PRINCIPAL DISPLAY PANEL - Sinu Inhaler
NEW
NDC 13709-281-07
NeilMed®
Sinu Inhaler™
Nasal Decongestant
_______________________
Levmetamfetamine - Nasal Decongestant
Temporary
Relief of Nasal Congestion due to:
Upper Respiratory Allergies
Cold • Hay Fever
Net Wt 0.007 OZ (198 mg) See Drug Facts
6031-ENU-US-rev1-PP20140409zm