Active ingredients (in each gram)
Bacitracin zinc equivalent to 400 units bacitracin
Neomycin sulfate equivalent to 3.5 mg of neomycin base
Polymyxin B 5,000 units as polymyxin B sulfate
Warnings
For external use only.
Do not use
- •
- if you are allergic to any of the ingredients
- •
- in the eyes
- •
- over large areas of the body
Directions
- •
- clean the affected area
- •
- apply a small amount of this product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- •
- may be covered with a sterile bandage
Inactive ingredients
cocoa butter, cottonseed oil, olive oil, sodium pyruvate, tocopheryl acetate, white petrolatum
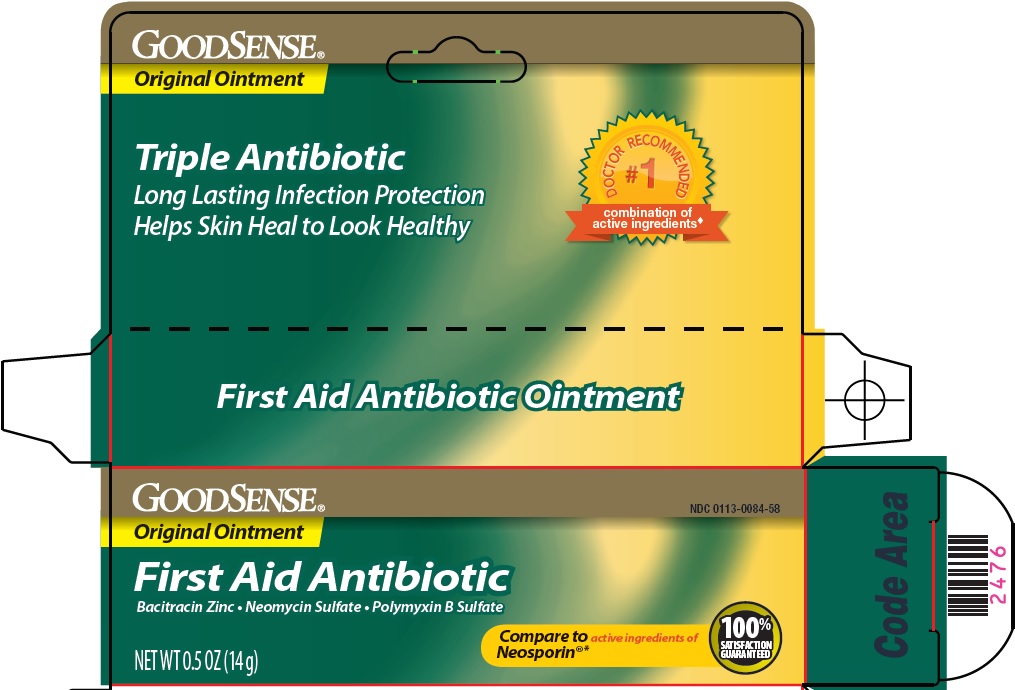
Principal Display Panel
Original Ointment
Triple Antibiotic
Long Lasting Infection Protection
Helps skin heal to look healthy
DOCTOR RECOMMENDED #1 combination of active ingredients
Original Ointment
First Aid Antibiotic
Bacitracin Zinc – Neomycin Sulfate – Polymyxin B Sulfate
Compare to active ingredients of Neosporin®
100% SATISFACTION GUARANTEED
NET WT 0.5 OZ (14 g)