Uses
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- sneezing
- itchy, watery eyes
- runny nose
- itching of the nose or throat
- temporarily relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies
- reduces swelling of nasal passages
- temporarily relieves sinus congestion and pressure
- temporarily restores freer breathing through the nose
Warnings
Do not use
- if you have ever had an allergic reaction to this product or any of its ingredients
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- heart disease
- thyroid disease
- high blood pressure
- diabetes
- trouble urinating due to an enlarged prostate gland
- liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product do not take more than directed. Taking more than directed may cause drowsiness.
Directions
- do not divide, crush, chew or dissolve the tablet
| adults and children 12 years and over | 1 tablet daily with a full glass of water; not more than 1 tablet in 24 hours |
| children under 12 years of age | ask a doctor |
| consumers with liver or kidney disease | ask a doctor |
Other information
- store between 20° to 25°C (68° to 77°F)
- protect from light and store in a dry place
Inactive ingredients
black iron oxide, candelilla wax powder, Inactive ingredients colloidal silicon dioxide, glyceryl monostearate, hypromellose, lactose monohydrate, magnesium stearate, polyethylene glycol, polysorbate 80, propylene glycol, sodium lauryl sulfate, talc, titanium dioxide
PRINCIPAL DISPLAY PANEL - 10 Tablet Blister Pack Carton
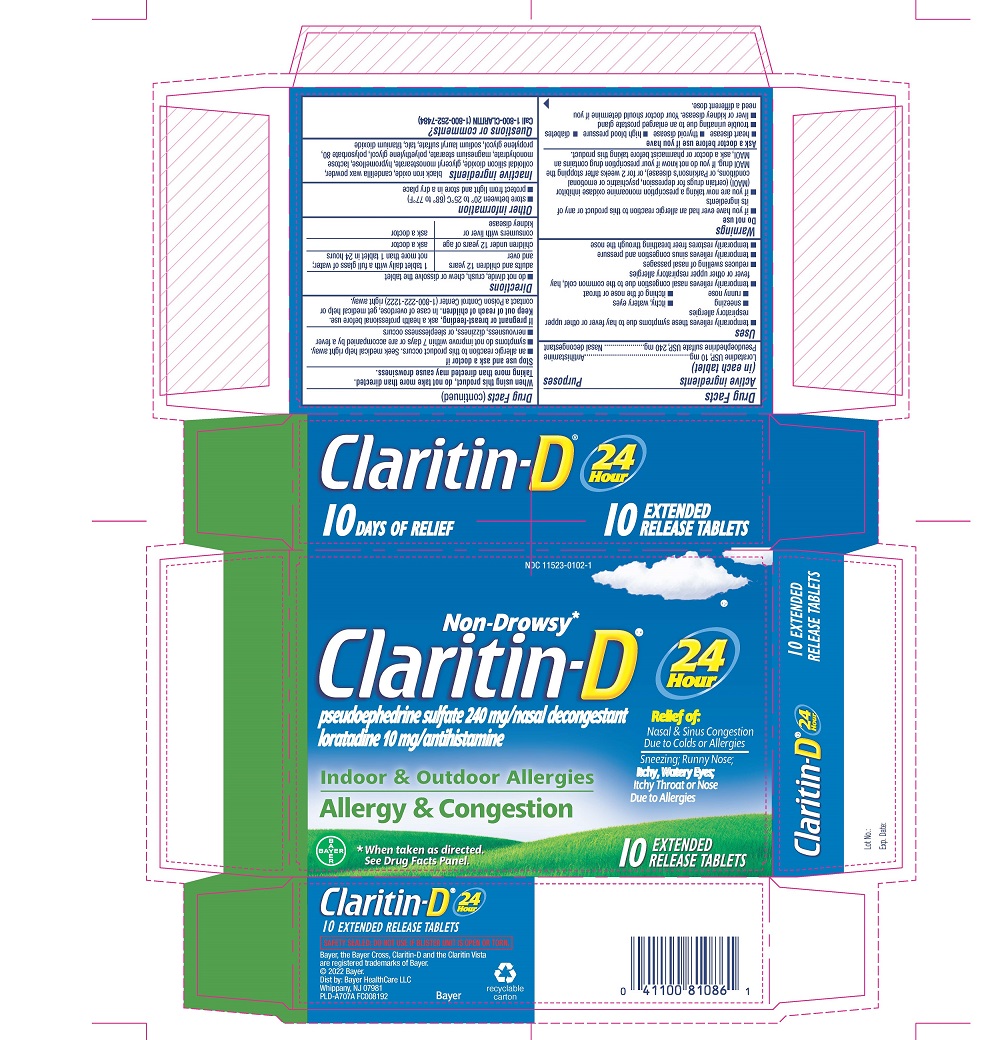
Non-Drowsy*
Claritin-D ®
pseudoephedrine sulfate 240 mg/nasal decongestant
loratadine 10 mg/antihistamine
Indoor & Outdoor Allergies
Allergy & Congestion
24
Hour
Relief of:
Nasal & Sinus Congestion
Due to Colds or Allergies
Sneezing; Runny Nose;
Itchy, Watery Eyes;
Itchy Throat or Nose
Due to Allergies
* When taken as directed. See Drug Facts Panel.
5
EXTENDED
RELEASE TABLETS