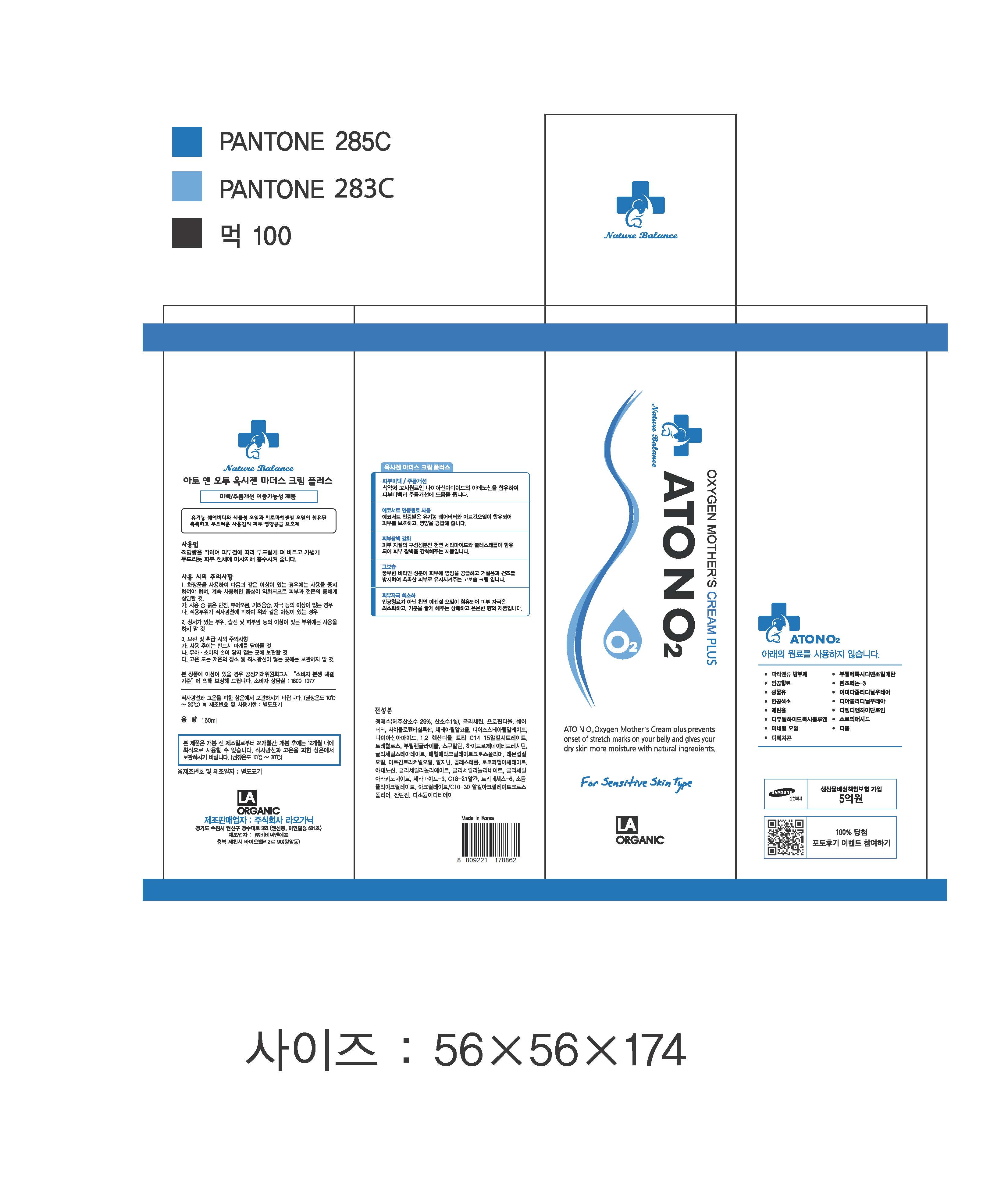
Take an appropriate amount and gently apply onto skin. Massage the whole area by lightly patting to let it fully absorb the product.
- For external use only
- When using this product
1. If any one of following symptoms occurs when using cosmetics, stop using it and consult a dermatologist.
A. red spot, swelling, itching, stimulus
B. symptoms mentioned above are caused by sunlight.
2. Do not apply on skin where there is wound, eczema, or irritation.
3. Storage and cautions for handling
A. Close stopper after using.
B. Keep out of reach of children.
C. Keep away from high or low temperature and direct sunlight.
4. This is not a medicinal product. Made of 100% cosmetic ingredients, it is a sensitive-skin care product for all skin types.