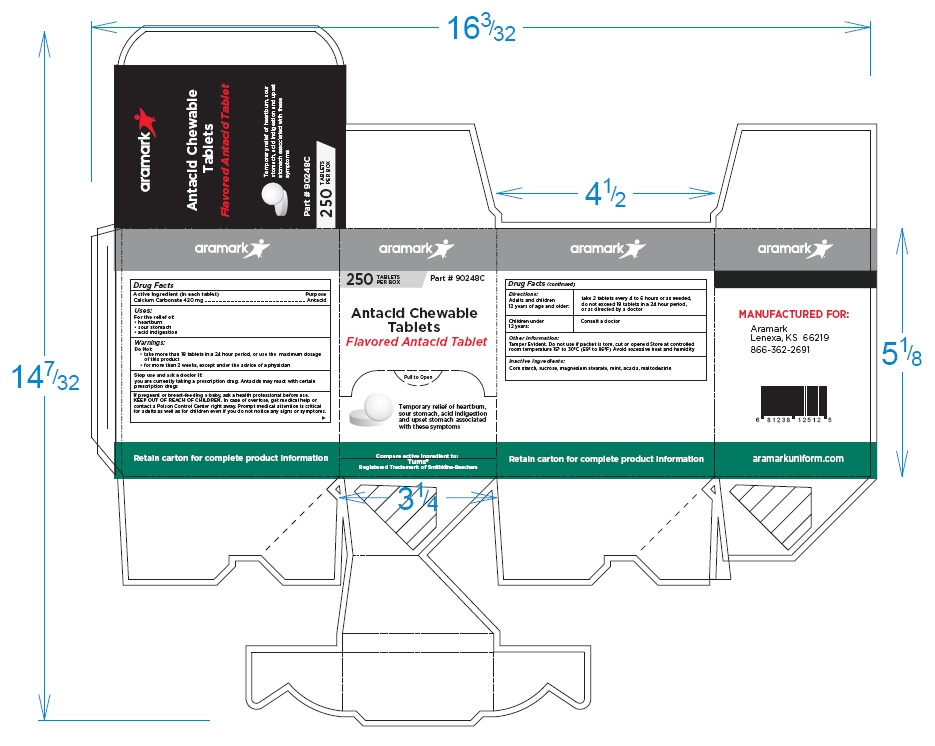
Warnings:Do Not:• take more than 18 tablets in a 24 hour period, or use the maximum dosage of this
product
• for more than 2 weeks, except under the advice of a physician
Stop use and ask a doctor if:
you are currently taking a prescription drug. Antacids may react with certain prescription
drugs
Keep out of reach of children In case of overfose, get medical help or contact a Poison Control
Center right away. Prompt medical attention is critical for adults as well as for children
even if you do not notice any signs or symptoms.
| Directions:
Adults and children 12 years of age and older: | do not exceed 18 tablets in a 24 hour period, or as directed by a doctor |
| Children under 12 years: | Consult a doctor |
Other information:
Tamper Evident. Do not use if packet is torn, cut or opened Store at controlled
room temperature 15º to 30ºC (59º to 86ºF) Avoid excessive heat and humidity
Package Labeling
aramark
100TABLETS Part # 90233B
PER BOX
Antacid Chewable Tablets
Flavored Antacid Tablet
Temporary relief of heartburn,
sour stomach, acid indigestion
and upset stomach associated
with these symptoms
Compare active ingredient to:
Tums® aramarkuniform.com
Registered Trademark of Smithkline-Beecham
MANUFACTURED FOR:
Aramark
Lenexa, KS 66219
866-362-2691
aramarkuniform.com
Retain carton for complete product information
100 Tablet Box

250 Tablet Box
2-Tablet Packet

res