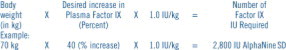DESCRIPTION
Coagulation Factor IX (Human), AlphaNine® SD, is a purified, solvent detergent treated, virus filtered preparation of Factor IX derived from human plasma.1 It contains a minimum of 150 IU Factor IX/mg protein; levels of Factor VII (proconvertin), Factor II (prothrombin) and Factor X (Stuart-Prower Factor) which are below the limit of detection (less than 0.04 Factor VII unit, less than 0.05 Factor II unit, and less than 0.05 Factor X unit per IU Factor IX). AlphaNine SD is a sterile, lyophilized preparation intended for intravenous administration only. Each vial is a single dose container.
AlphaNine SD is labeled with the Factor IX potency expressed in International Units (IU). AlphaNine SD contains not more than (NMT) 0.04 unit of heparin, NMT 0.2 mg of dextrose, NMT 1.0 μg polysorbate 80 and NMT 0.10 μg tri(n-butyl) phosphate/IU of Factor IX. Contains no preservatives.
CLINICAL PHARMACOLOGY
AlphaNine SD is a purified formulation of Factor IX containing not less than 150 IU Factor IX activity/mg of total protein.2 AlphaNine SD contains non-therapeutic levels of Factor II, Factor VII and Factor X.
Thrombogenicity of AlphaNine SD in animals is markedly lower than that of Factor IX Complex, Profilnine® Heat-Treated. Five lots of AlphaNine SD (three lots of non-virus filtered product and two lots of virus filtered product) failed to show any evidence of thrombogenicity when tested directly in the Wessler rabbit stasis model for thrombogenicity3-6 at a dose of 200 IU Factor IX/kg body weight. When various lots of AlphaNine SD were further tested at doses between 300 and 650 IU Factor IX/kg, only 5 out of 40 animals (12.5%) showed evidence of thrombus formation (Wessler scores of +1, +2, +1, +1, +1 out of +4 maximum). In comparison, Factor IX Complex concentrate, Profilnine, was thrombogenic in 100% of the animals tested at a dose of 100 IU Factor IX/kg.
At a dose of 200 IU Factor IX/kg body weight in a porcine model, the heptane heat-treated formulation of this product (AlphaNine) showed little evidence of disseminated intravascular coagulation (DIC) following infusion.7 This model exhibited no depletion of coagulation factors, a minimal increase in fibrin monomer (+1 in protamine test), a slight temporary decrease in platelet counts, and no evidence of intravascular coagulation upon gross autopsy.8 In contrast, Harrison, et al., report that all Factor IX Complex concentrates studied in the same porcine model were thrombogenic at doses between 50 and 100 IU of Factor IX/kg animal weight.9
A clinical evaluation of AlphaNine SD half-life and recovery characteristics was performed. A total of 18 patients with severe to moderate hemophilia B each received a single infusion of 40 to 50 IU Factor IX/kg body weight of AlphaNine SD. Following the administration of AlphaNine SD, the mean half-life of Factor IX observed was approximately 21 hours.2 This half-life value was computed using the biphasic linear regression model recommended by the International Society of Thrombosis and Haemostasis.10 The half-life obtained for the solvent detergent treated product is comparable to that of AlphaNine (approximately 19 hours) as well as the range of 18 to 36 hours reported for Factor IX Complex preparations.11 The mean recovery observed in clinical trials was approximately 48% and was comparable to that of AlphaNine (approximately 51%).2
A clinical trial was conducted using the heptane heat-treated product, AlphaNine, to evaluate the efficacy of the product in providing hemostatic protection during and after surgery in 13 patients with hemophilia B. The types of surgical procedures performed included bilateral knee replacement (1), total knee replacement with synovectomy (2), hip replacement (1), below the knee amputation (1), herniorrhaphy (2), hemorrhoidectomy (1), rhinoplasty (2), oral surgery (2) and Hickman catheter insertion with temporalis muscle transfer (1). Presurgery doses ranged from 30.1 to 65.0 IU Factor IX/kg; postsurgery replacement therapy doses ranged from approximately 9.4 to 52.0 IU Factor IX/kg. The number of postsurgery days of treatment ranged from 1 to 23; the number of postsurgery infusions ranged from 2 to 26. No bleeding episodes were reported and hemostasis was maintained during the course of postsurgery therapy. None of the hematologic parameters examined (hematocrit, partial thromboplastin time, prothrombin time, fibrinogen/fibrin degradation products, fibrin monomers, D-dimers and platelet counts) provided any evidence that AlphaNine possessed thrombogenic potential.12
A randomized crossover study with 11 hemophilia B patients was conducted with the heptane heat-treated version of the product, AlphaNine, to determine whether an infusion of AlphaNine caused less activation of the hemostatic system than the Factor IX Complex concentrate preparation, Profilnine Heat-Treated. Each subject received a single infusion of either AlphaNine or Profilnine Heat-Treated for the treatment of a bleeding episode, at a dose of 50 IU Factor IX/kg body weight. Each subject received the other Factor IX concentrate for the treatment of a subsequent bleeding episode, separated by an interval of not less than 10 days. The level of prothrombin fragment 1+2 (F1+2) is a sensitive index of the cleavage of prothrombin by activated Factor X. The level of fibrinopeptide A (FPA) released into the plasma measures the activity of thrombin on fibrinogen in the formation of fibrin. Following infusion of Factor IX Complex, statistically significant increases in F1+2 and in FPA were detected at all monitored time points (15, 60, 90, 120 and 240 minutes postinfusion). The statistically significant elevation in these two hemostatic parameters indicates increased activation of the coagulation cascade. Administration of AlphaNine resulted in no increase in F1+2 at any monitored time points, and a statistically non-significant increase in FPA at 15, 60, and 90 minutes following infusion. Only at 120 and 240 minutes after infusion of AlphaNine were statistically significant increases in FPA levels detected. These results suggest that the infusion of a high purity factor IX, such as AlphaNine, may result in a lower level of activation of the coagulation cascade than does Factor IX Complex.13
The ability of the manufacturing process to inactivate and eliminate virus from the Coagulation Factor IX (Human) products was evaluated at key stages in the process (see Table 1). Known amounts of different viruses were added to samples obtained prior to those steps most likely to reduce virus load (DEAE Chromatography, Solvent Detergent, Dual Affinity Chromatography and nanofiltration) in the AlphaNine and AlphaNine SD processes to determine the level of viral inactivation/elimination of these specific steps in the process.
| **Porcine NT=Not tested NLT=Not less than *Lower 95% confidence interval | ||||||||
| Process Step | Virus Reduction (log10) | |||||||
| Sindbis | VSV | HIV-1 | HIV-2 | Parvo** | EMC | Reo | HAV | |
| DEAE Chromatography | 1.4 | NT | NT | NT | 1.5* | NT | NT | NT |
| Solvent- Detergent | NLT 5.3 | NLT 4.9 | NLT 12.2 | 6.0 | NT | NT | NT | NT |
| Dual Affinity Chromatography | 4.7 | NT | NT | NT | 2.2* | NT | NT | NT |
| Nanofiltration | NT | NT | NT | NT | 3.6 | 3.4 | 4.1 | ≥ 4.4 |
The retrovirus known as human immunodeficiency virus (HIV) has been identified as a causative agent of Acquired Immunodeficiency Syndrome (AIDS) and has been shown to be transmissible via blood or blood products. The solvent detergent process used in the manufacture of AlphaNine SD, was shown to inactivate greater than 12.2 logs of HIV-1 when the retrovirus was intentionally added to product samples under laboratory evaluation (as measured by virus antigen capture and reverse transcriptase assays). In addition, this process was shown to inactivate 6 logs of HIV-2 (as measured by reverse transcriptase assays) when the retrovirus was intentionally added to product samples.2 In an on going efficacy and safety study of 26 patients, no subjects tested positive for HIV or viral hepatitis in relation to the investigation drug.2
In order to assess the ability of the solvent detergent treatment process to inactivate other viruses such as hepatitis B and C virus, the inactivation of the model viruses, Sindbis virus, a model virus for hepatitis C virus, and vesicular stomatitis virus (VSV), a model RNA virus for lipid enveloped viruses, by solvent detergent treatment was studied. Prior to solvent detergent treatment, samples were inoculated with a titer of either Sindbis or VSV. The results demonstrated that a minimum of 5.3 logs of Sindbis and a minimum of 4.9 logs of VSV were inactivated after 180 minutes of incubation with solvent detergent (when compared to an untreated control). It should be noted that the incubation time in the actual AlphaNine SD process is twice (360 minutes total) that used in the model virus studies.
The ability of the AlphaNine SD process to eliminate virus, by physically partitioning virus from product, was evaluated at key stages of the manufacturing process. Studies were performed using a lipid-enveloped model virus (Sindbis) and non-lipid model viruses (porcine parvovirus, encephalomyocarditis virus, and reovirus). Known amounts of these viruses were added to samples obtained from the AlphaNine SD process. The amount of virus removed at each subsequent purification step was then determined by plaque assay.
Addition of Sindbis or porcine parvovirus prior to Factor IX Complex adsorption by DEAE chromatography showed this step to eliminate 1.4 logs of Sindbis and 1.5 logs (95% confidence interval: 1.51-2.33) of added porcine parvovirus. When Sindbis or parvovirus was introduced into the process after the barium citrate precipitation step of the AlphaNine SD process, the subsequent dual affinity chromatography step was found to eliminate 4.7 logs of Sindbis and 2.2 logs (95% confidence interval: 2.25-2.75) of added parvovirus. When parvovirus, encephalomyocarditis virus (EMC), or Reovirus was introduced into the process after the dual affinity chromatography step, the subsequent nanofiltration step of the AlphaNine SD process was found to eliminate 3.6 logs of parvovirus, 3.4 logs of EMC and 4.1 logs of added Reovirus. The studies mentioned above indicate that the manufacturing process of AlphaNine SD is capable of reducing viruses by approximately 6 logs, in addition to virus reduction achieved by the solvent detergent process.14 In another study, the nanofiltration step removed ≥ 4.4 logs of hepatitis A virus (HAV), a non-lipid enveloped virus. Table 1 summarizes the reduction factors obtained for each virus when individual steps in the manufacturing process for AlphaNine SD were validated for virus removal/inactivation.
INDICATIONS AND USAGE
AlphaNine SD is indicated for the prevention and control of bleeding in patients with Factor IX deficiency due to hemophilia B. AlphaNine SD contains low, non-therapeutic levels of Factors II, VII, and X, and, therefore, is not indicated for the treatment of Factor II, VII or X deficiencies. This product is also not indicated for the reversal of coumarin anticoagulant-induced hemorrhage, nor in the treatment of hemophilia A patients with inhibitors to Factor VIII.
WARNINGS
Because Coagulation Factor IX (Human), AlphaNine SD is made from pooled human plasma, it may carry a risk of transmitting infectious agents, e.g., viruses, and theoretically, the Creutzfeldt-Jakob disease (CJD) agent. Stringent procedures designed to reduce the risk of adventitious agent transmission have been employed in the manufacture of this product, from the screening of plasma donors and the collection and testing of plasma to the application of viral elimination/reduction steps such as column chromatography, solvent detergent treatment and nanofiltration in the manufacturing process. Despite these measures, such product can potentially transmit disease, therefore the risk of infectious agents cannot be totally eliminated. The physician should weigh the risks and benefits of the use of this product and should discuss these with the patient.
Individuals who receive infusions of blood or plasma products may develop signs and/or symptoms of some viral infections. Scientific opinion encourages hepatitis B and hepatitis A vaccinations at birth or diagnosis for patients with hemophilia.
Incidences of thrombosis or disseminated intravascular coagulation (DIC), have been reported following administration of Factor IX Complex concentrates which contain high amounts of Factor II, VII and X.
Following administration of Coagulation Factor IX (Human), AlphaNine SD in surgery patients and individuals with known liver disease, the physician should closely observe the patient for signs or symptoms of potential disseminated intravascular coagulation (DIC). Continued administration of the product should be left to the discretion of the physician.
Allergic type hypersensitivity reactions, including anaphylaxis, have been reported for all factor IX products. Frequently these events have occurred in close temporal association with the development of factor IX inhibitors. Patients should be informed of the early symptoms and signs of hypersensitivity reactions, including hives, generalized urticaria, angioedema, chest tightness, dyspnea, wheezing, faintness, hypotension, tachycardia and anaphylaxis. Patients should be advised to discontinue use of the product and contact physician and/or seek immediate emergency care, depending on the severity of the reactions, if any of these symptoms occur.
Nephrotic syndrome has been reported following attempted immune tolerance induction with factor IX products in Hemophilia B patients with factor IX inhibitors and a history of severe allergic reactions to Factor IX. The safety and efficacy of using AlphaNine SD in attempted immune tolerance induction has not been established.
In Previously Untreated Patients (PUPs), it is possible that anaphylaxis may occur after a median exposure of eleven (11) days.15 It is recommended that these patients are monitored closely between the tenth and twentieth exposure day.
PRECAUTIONS
General
In order to minimize the possibility of thrombogenic complications, dosing guidelines should be strictly followed. Refer to “Dosage and Administration” section for recommended amount of product to be administered.
AlphaNine SD should not be administered at a rate exceeding 10 mL/minute. Rapid administration may result in vasomotor reactions.
Nursing personnel and others who administer this material should exercise appropriate caution in handling due to the risk of exposure to viral infection.
Discard any unused contents into the appropriate safety container. Discard administration equipment after single use into the appropriate safety container. Do not resterilize components.
Information for Patients
Patients should be informed of the early symptoms and signs of hypersensitivity reaction, including hives, generalized urticaria, chest tightness, dyspnea, wheezing, faintness, hypotension, and anaphylaxis. Patients should be advised to discontinue use of the product and contact their physician and/or seek immediate emergency care, depending on the severity of the reaction, if these symptoms occur.
Some viruses, such as parvovirus B19 or hepatitis A, are particularly difficult to remove or inactivate at this time. Parvovirus B19 may most seriously affect sero-negative pregnant women, or immunocompromised individuals. The majority of parvovirus B19 and hepatitis A infections are acquired by environmental (natural) sources.
Preliminary information suggests a relationship may exist between the presence of major deletion mutations in the Factor IX gene and an increased risk of inhibitor formation and of acute hypersensitivity reactions. Patients known to have major deletion mutations of the Factor IX gene should be observed closely for signs and symptoms of acute hypersensitivity reactions, particularly during the early phases of initial exposure to product.
Pregnancy
Animal reproduction studies have not been conducted with AlphaNine SD. It is also not known whether AlphaNine SD can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. AlphaNine SD should be given to a pregnant woman only if clearly indicated.
Pediatric Use
Clinical trials for safety and effectiveness in pediatric patients 16 years of age and younger have not been conducted. Across a well controlled half-life and recovery clinical trial in patients previously treated with Factor IX concentrates of Hemophilia B, the three pediatric patients receiving AlphaNine SD (solvent detergent treated) responded similarly when compared with 15 adult patients.2 In an ongoing safety and efficacy clinical trial in patients not previously treated with Factor IX concentrates for Hemophilia B, 21 pediatric patients received AlphaNine SD (solvent detergent treated) responded similarly when compared with the five adult patients above the age of 16 years. Adverse events were similar in this group compared to the patients above the age of 16 years. Anecdotal evaluation of the results indicates no safety and efficacy differences between pediatric and adult populations.
ADVERSE REACTIONS
The administration of plasma preparations may cause allergic reactions, mild chills, nausea or stinging at the infusion site. For most reactive individuals, slowing the infusion rate relieves the symptoms. For those highly reactive individuals, a different lot may be satisfactory.
Adverse reactions, characterized by either thrombosis or disseminated intravascular coagulation (DIC), have been reported following administration of Factor IX Complex concentrates. Patients who receive Coagulation Factor IX (Human), AlphaNine SD, following operation, or those with known liver disease, should be kept under close observation for potential signs or symptoms of intravascular coagulation. Continued administration should be left to the discretion of the physician.
In the clinical study that compared the in vivo half-life and recovery of AlphaNine SD and HT products, no adverse events were associated with 18 infusions of AlphaNine SD administered to 18 individuals with severe to moderate hemophilia B.2 Short term safety of the earlier version of this product, AlphaNine, was demonstrated by an absence of adverse events after 225 infusions of this product were received by 31 patients participating in three clinical trials. In the clinical trial to evaluate efficacy of AlphaNine in providing hemostatic protection during and after surgery, 13 patients received a total of 370,655 IU of AlphaNine. In 208 total infusions, each patient received approximately 15,000 IU (range 3,295 to 52,200 IU Factor IX) in an average of 16 infusions (range 2 to 26 infusions). Results from this study showed no bleeding episodes during the course of postsurgery therapy. There was no hematological evidence (measured by hematocrit, partial thromboplastin time, prothrombin time, fibrinogen/fibrin degradation products, fibrin monomers, D-dimers and platelet counts) of thrombogenicity.12
To report SUSPECTED ADVERSE REACTIONS, contact Grifols at 1-888-GRIFOLS (1-888-474-3657) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DOSAGE AND ADMINISTRATION
For adult usage:
AlphaNine SD should be administered intravenously promptly following reconstitution. Administration of AlphaNine SD within three hours after reconstitution is recommended to avoid the potential ill effect of any inadvertent bacterial contamination occurring during reconstitution. Discard any unused contents into the appropriate safety container.
Each vial of AlphaNine SD is labeled with the total units expressed as International Units (IU) of Factor IX, which is referenced to the WHO International Standard. One unit approximates the activity in one mL of pooled normal human plasma.
The amount of AlphaNine SD required to establish hemostasis will vary with each patient and depend upon the circumstances. The following formula may be used as a guide in determining the number of units to be administered.16
In clinical practice there is variability between patients and their clinical response. Therefore, the Factor IX level of each patient should be monitored frequently during replacement therapy.
For pediatric usage: See PRECAUTIONS
| Type of Hemorrhage or Surgical Procedure | Examples | Treatment Guidelines |
| Minor Hemorrhages | Bruises, cuts or scrapes, uncomplicated joint hemorrhage | FIX levels should be brought to at least 20-30% (20-30 IU FIX/kg/twice daily) until hemorrhage stops and healing has been achieved (1-2 days).17,18,19 |
| Moderate Hemorrhages | Nose bleeds, mouth and gum bleeds, dental extractions, hematuria | FIX levels should be brought to 25-50% (25-50 IU FIX/kg/twice daily) until healing has been achieved (2-7 days, on average).17,18,19,20,21 |
| Major Hemorrhages | Joint and muscle hemorrhages (especially in the large muscles), major trauma, hematuria, intracranial and intraperitoneal bleeding | FIX levels should be brought to 50% for at least 3-5 days (30-50 IU FIX/kg/twice daily). Following this treatment period, FIX levels should be maintained at 20% (20 IU FIX/kg/twice daily) until healing has been achieved. Major hemorrhages may require treatment for up to 10 days.17,18,19,20,21 |
| Surgery | Prior to surgery, FIX should be brought to 50-100% of normal (50-100 IU FIX/kg/twice daily). For the next 7 to 10 days, or until healing has been achieved, the patient should be maintained at 50-100% FIX levels (50-100 IU FIX/kg/twice daily).17,18,19,20,21 |
Dosing requirements and frequency of dosing is calculated on the basis of an initial response of 1% FIX increase achieved per IU of FIX infused per kg body weight and an average half-life for FIX of 18 hours. If dosing studies have revealed that a particular patient exhibits a lower response, the dose should be adjusted accordingly.
For pediatric usage: See PRECAUTIONS
RECONSTITUTION
Use Aseptic Technique
- Warm diluent (Sterile Water for Injection, USP) and concentrate (AlphaNine SD) to at least room temperature (but not above 37 °C).
- Remove the plastic flip off cap from the diluent vial.
- Gently swab the exposed stopper surface with a cleansing agent such as alcohol trying to avoid leaving any excess cleansing agent on the stopper.
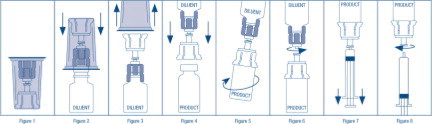
- Open the Mix2Vial® package by peeling away the lid (Figure 1). Leave the Mix2Vial in the clear outer packaging.
- Place the diluent vial upright on an even surface and hold the vial tight and pick up the Mix2Vial in its clear outer packaging. Holding the diluent vial securely, push the blue end of the Mix2Vial vertically down through the diluent vial stopper (Figure 2).
- While holding onto the diluent vial, carefully remove the clear outer packaging from the Mix2Vial set, ensuring the Mix2Vial remains attached to the diluent vial (Figure 3).
- Place the product vial upright on an even surface, invert the diluent vial with the Mix2Vial attached.
- While holding the product vial securely on a flat surface, push the clear end of the Mix2Vial set vertically down through the product vial stopper (Figure 4). The diluent will automatically transfer out of its vial into the product vial. (NOTE: If the Mix2Vial is connected at an angle, the vacuum may be released from the product vial and the diluent will not transfer into the product vial.)
- With the diluent and product vials still attached to the Mix2Vial, gently swirl the product vial to ensure the product is fully dissolved (Figure 5). Reconstitution requires less than 5 minutes. Do not shake the vial.
- Disconnect the Mix2Vial into two separate pieces (Figure 6) by holding each vial adapter and twisting counterclockwise. After separating, discard the diluent vial with the blue end of the Mix2Vial.
- Draw air into an empty, sterile syringe. Keeping the product vial upright with the clear end of the Mix2Vial attached, screw the disposable syringe onto the luer lock portion of the Mix2Vial device by pressing and twisting clockwise. Inject air into the product vial.
- While keeping the syringe plunger depressed, invert the system upside down and draw the reconstituted product into the syringe by pulling the plunger back slowly (Figure 7).
- When the reconstituted product has been transferred into the syringe, firmly hold the barrel of the syringe and the clear vial adapter (keeping the syringe plunger facing down) and unscrew the syringe from the Mix2Vial (Figure 8). Hold the syringe upright and push the plunger until no air is left in the syringe. Attach the syringe to a venipuncture set.
- NOTE: If the same patient is to receive more than one vial of concentrate, the contents of two vials may be drawn into the same syringe through a separate unused Mix2Vial set before attaching to the venipuncture set.
- Use the prepared drug as soon as possible within three hours after reconstitution.
- After reconstitution, parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. When reconstitution procedure is strictly followed, a few small particles may occasionally remain. The Mix2Vial set will remove particles and the labeled potency will not be reduced.
- Discard all administration equipment after use into the appropriate safety container. Do not reuse.
HOW SUPPLIED
AlphaNine SD is supplied in sterile, lyophilized form in single dose vials accompanied by 10 mL diluent (Sterile Water for Injection, USP). Factor IX activity, expressed in International Units (IU) which is referenced to WHO International Standard, is stated on the label of each concentrate vial. AlphaNine SD is packaged with a Mix2Vial filter transfer set for use in administration.
It is available in the following potencies, and the product is also color coded based upon assay on the carton and vial label as follows:
| Potency | NDC | Assay Color Code |
| 500 IU FIX/10 mL single dose vial | 68516-3610-2 or 68516-3607-2 | 500 IU FIX Range - gray box |
| 1000 IU FIX/10 mL single dose vial | 68516-3611-2 or 68516-3608-2 | 1000 IU FIX Range - green box |
| 1500 IU FIX/10 mL single dose vial | 68516-3612-2 or 68516-3609-2 | 1500 IU FIX Range - blue box |
STORAGE
AlphaNine SD is stable for three years, up to the expiration date printed on its label, provided that the storage temperature is between 2 and 8 °C (36 and 46 °F). Do not freeze to prevent damage to diluent vial. May be stored at room temperature not to exceed 30 °C for 1 month. When removed from refrigeration, record the date removed on the space provided on the carton.
Rx only
REFERENCES
- Plasma Fraction Purification Serial No. 902.155 Patent issued.
- Data on file at Grifols Biologicals LLC
- Giles, A.R., Johnston, M., Hoogendoorn, H., Blajchman, M. & Hirsch, J. The Thrombogenicity of Prothrombin Complex Concentrates: I. The Relationship Between In Vitro Characteristics and In Vivo Thrombogenicity in Rabbits. Thromb Res 17:353-366, 1980.
- Kingdon, H.S., Lundblad, R.L., Veltkamp, J.J. & Aronson, D.L. Potentially Thrombogenic Materials in Factor IX Concentrates. Thromb Diath Haemorrh (Stuttg) 33:617-631, 1975.
- Prowse, C.V. & Williams, A.E. A Comparison of the In Vitro and In Vivo Thrombogenic Activity of Factor IX Concentrate Using Stasis (Wessler) and Non-Stasis Rabbit Models. Thromb Hemostas 44:81-86, 1980.
- Wessler, S., Reimer, S.M. & Sheps, M.C. Biologic Assay of a Thrombosis-Inducing Activity in Human Serum. J Appl Physiol 14:943-946, 1959.
- Herring, S.W. & Heldebrant, C.M. Heat-Treated Pure Factor IX. Proc 5th Int Symp HT, 1986, pp. 151-158.
- Herring, S.W., Abildgaard, C., Shitanishi, K.T., Harrison, J., Gendler, S. & Heldebrant, C.M. Human Coagulation Factor IX Assessment of Thrombogenicity in Animal Models and Viral Safety. J. Lab. Clin Med. 121: 394-405, 1993.
- Harrison, J., Abildgaard, C., Lazerson, J., Culbertson, R. & Anderson, G. Assessment of Thrombogenicity of Prothrombin Complex Concentrates in a Porcine Model. Throm Res 38(2): 173-188, 1985.
- Lee, M., Poon, W., & Kingdon, H. A Two-Phase Linear Regression Model for Biologic Half-Life Data. J Lab Clin Med 115(6): 745-748, 1990.
- White, G.C., Lundblad, R.L., & Kingdon, H.S. Prothrombin Complex Concentrates: Preparation, Properties, and Clinical Uses. Curr Topic in Hematol 2:203-244, 1979.
- Goldsmith, J.C., Kasper, C.K., et al. Coagulation Factor IX: Successful Surgical Experience With a Purified Factor IX Concentrate. Amer J Hematol 40: 210-215, 1992.
- Mannucci, P.M., Bauer, K.A., Gringeri, A., Barzegar, S., Bottasso, B., Simoni L. & Rosenberg, R.D. Thrombin Generation Is Not Increased in the Blood of Hemophilia B Patients After the Infusion of a Purified Factor IX Concentrate. Blood 76(12): 2540-2545, December 15, 1990.
- Herring, S., Peddada, L., Shitanishi, K., Chavez, D., Chio, A., & Heldebrant, C. Elimination of Virus During Manufacture of a Coagulation Factor IX Concentrate. Abstract presented at XIX International Congress World Federation of Hemophilia, Washington, DC, USA, August 14-19, 1990.
- Warrier, I., Ewenstein, B.M., Koerper, M.A., Shapiro, A., Key, N., DiMichele, D., Miller, R.T., Pasi, J., Rivard, G.E., Sommer, S.S., Katz, J., Bergmann, F., Ljung, R., Petrini, P., Lusher, J.M. Journal of Pediatric Hematology/Oncology 19(1):23-27, 1997.
- Zauber, N.P. & Levine, J. Factor IX Levels in Patients with Hemophilia B (Christmas Disease) Following Transfusion with Concentrates of Factor IX or Fresh Frozen Plasma (FFP). Medicine 56:213-224, 1977.
- Nillson, I.M.: Hemorrhagic and Thrombotic Diseases: London, John Wiley and Sons, 1974.
- Roberts, H.R. and Eberst, M.E.: Current Management of Hemophilia B. Hematology/Oncology Clinics of North America 7(6): 1269-1280, 1993.
- Roberts, H.R. and Gray, T.F.: Clinical Aspects of Hemophilia B. In Hematology: Basic Principles and Practice, 2nd Edition, pp 1678-1685, Churchill Livingston.
- Hedner, U. and Davie, E.W.: In “Hemostasis and Thrombosis: Basic Principles and Clinical Practice”, eds. Colman, R.W., Hirsh, J., Marder, V.J., Salzman, E.W., 2nd Edition, Philadelphia, J.B. Lippincot Co, 1987.
- Levin, P.H.: In “Hemostasis and Thrombosis: Basic Principles and Clinical Practice”, eds. Colman, R.W., Hirsh, J., Marder, V.J., Salzman, E.W., 2nd Edition, Philadelphia, J.B, Lippincot Co, 1987.
Manufactured by:
Grifols Biologicals LLC
5555 Valley Boulevard
Los Angeles, CA 90032, U.S.A.
U.S. License No. 1694
DATE OF REVISION: November 2022
3063702
Principal Display Panel – 500 IU Vial Label
NDC 68516-3604-2 500 IU FIX Range
Coagulation Factor IX (Human)
AlphaNine® SD
Solvent Detergent Treated / Virus
Filtered
Storage: Store between 2 and 8 °C.
May be stored at room temperature
not to exceed 30 °C for 1 month.
Rx Only. Single dose container for
intravenous administration only.
U.S. License No. 1694 10 mL
Instructions: Reconstitute with 10 mL
Sterile Water for Injection, USP.
Administer promptly after
reconstitution and do not refrigerate.
Discard unused contents. For
information on dosage and
directions for administration, see
accompanying pamphlet. Contains
no preservatives. The patient and
physician should discuss the risks
and benefits of this product.
Grifols Biologicals LLC
5555 Valley Boulevard,
Los Angeles, CA 90032, U.S.A.
Lot
EXP
IU FIX/Vial 3063701
Lot
IU FIX/Vial
Alphanine® SD
10 mL NDC 68516-3604-2

Principal Display Panel – 1000 IU Vial Label
NDC 68516-3605-2 1000 IU FIX Range
Coagulation Factor IX (Human)
AlphaNine® SD
Solvent Detergent Treated / Virus
Filtered
Storage: Store between 2 and 8 °C.
May be stored at room temperature
not to exceed 30 °C for 1 month.
Rx Only. Single dose container for
intravenous administration only.
U.S. License No. 1694 10 mL
Instructions: Reconstitute with 10 mL
Sterile Water for Injection, USP.
Administer promptly after
reconstitution and do not refrigerate.
Discard unused contents. For
information on dosage and
directions for administration, see
accompanying pamphlet. Contains
no preservatives. The patient and
physician should discuss the risks
and benefits of this product.
Grifols Biologicals LLC
5555 Valley Boulevard,
Los Angeles, CA 90032, U.S.A.
Lot
EXP
IU FIX/Vial 3063699
Lot
IU FIX/Vial
Alphanine® SD
10 mL NDC 68516-3605-2

Principal Display Panel – 1500 IU Vial Label
NDC 68516-3606-2 1500 IU FIX Range
Coagulation Factor IX (Human)
AlphaNine® SD
Solvent Detergent Treated / Virus
Filtered
Storage: Store between 2 and 8 °C.
May be stored at room temperature
not to exceed 30 °C for 1 month.
Rx Only. Single dose container for
intravenous administration only.
U.S. License No. 1694 10 mL
Instructions: Reconstitute with 10 mL
Sterile Water for Injection, USP.
Administer promptly after
reconstitution and do not refrigerate.
Discard unused contents. For
information on dosage and
directions for administration, see
accompanying pamphlet. Contains
no preservatives. The patient and
physician should discuss the risks
and benefits of this product.
Grifols Biologicals LLC
5555 Valley Boulevard,
Los Angeles, CA 90032, U.S.A.
Lot
EXP
IU FIX/Vial 3063697
Lot
IU FIX/Vial
Alphanine® SD
10 mL NDC 68516-3606-2

Principal Display Panel – 500 IU Carton Label
NDC 68516-3610-2 500 IU FIX Range
Coagulation Factor IX (Human)
AlphaNine® SD
Solvent Detergent Treated / Virus Filtered
Rx only
For Intravenous Administration 10 mL
GRIFOLS
Contents:
One vial Coagulation Factor IX (Human), AlphaNine®
SD, one vial 10 mL Sterile Water for Injection, USP,
one Mix2Vial® filter transfer set, and directions for
use.
For intravenous administration.
The reconstituted product contains not more than
0.04 unit of heparin, and not more than 0.2 mg of
dextrose, and not more than 1.0 µg polysorbate 80
and 0.10 µg tri(n-butyl) phosphate per IU of Factor IX.
Contains no preservatives.
Administer within three hours of reconstitution.
Discard unused contents.
GRIFOLS
Warning: This product is prepared from large
pools of human plasma. Human blood and its
components may transmit infectious agents. See
package insert, WARNINGS AND PRECAUTIONS.
Instructions: The patient and physician should
discuss the risks and benefits of this product.
For information on dosage and directions for
administration, see enclosed package insert.
Storage: Store between 2 and 8 °C. Avoid
freezing which might damage diluent bottle.
May be stored at room temperature not to exceed
30 °C for 1 month.
Single dose container for intravenous
administration only.
Date removed from refrigeration:
Grifols Biologicals LLC
5555 Valley Boulevard
Los Angeles, CA 90032, U.S.A.
U.S. License No 1694
GRIFOLS
GTIN 00368516361023
LOT XXXXXXXXXX
EXP DDMMMYYYY
SN XXXXXXXXXX XXXXXX
IU FI X/Vial XXXX
3063700
Principal Display Panel – 1000 IU Carton Label
NDC 68516-3611-2 1000 IU FIX Range
Coagulation Factor IX (Human)
AlphaNine® SD
Solvent Detergent Treated / Virus Filtered
Rx only
For Intravenous Administration 10 mL
GRIFOLS
Contents:
One vial Coagulation Factor IX (Human), AlphaNine®
SD, one vial 10 mL Sterile Water for Injection, USP,
one Mix2Vial® filter transfer set, and directions for
use.
For intravenous administration.
The reconstituted product contains not more than
0.04 unit of heparin, and not more than 0.2 mg of
dextrose, and not more than 1.0 µg polysorbate 80
and 0.10 µg tri(n-butyl) phosphate per IU of Factor IX.
Contains no preservatives.
Administer within three hours of reconstitution.
Discard unused contents.
Warning: This product is prepared from large
pools of human plasma. Human blood and its
components may transmit infectious agents. See
package insert, WARNINGS AND PRECAUTIONS.
Instructions: The patient and physician should
discuss the risks and benefits of this product.
For information on dosage and directions for
administration, see enclosed package insert.
Storage: Store between 2 and 8 °C. Avoid
freezing which might damage diluent bottle.
May be stored at room temperature not to exceed
30 °C for 1 month.
Single dose container for intravenous
administration only.
Date removed from refrigeration:
Grifols Biologicals LLC
5555 Valley Boulevard
Los Angeles, CA 90032, U.S.A.
U.S. License No 1694
GRIFOLS
GTIN 00368516361122
LOT XXXXXXXXXX
EXP DDMMMYYYY
SN XXXXXXXXXX XXXXXX
IU FI X/Vial XXXX
3063698
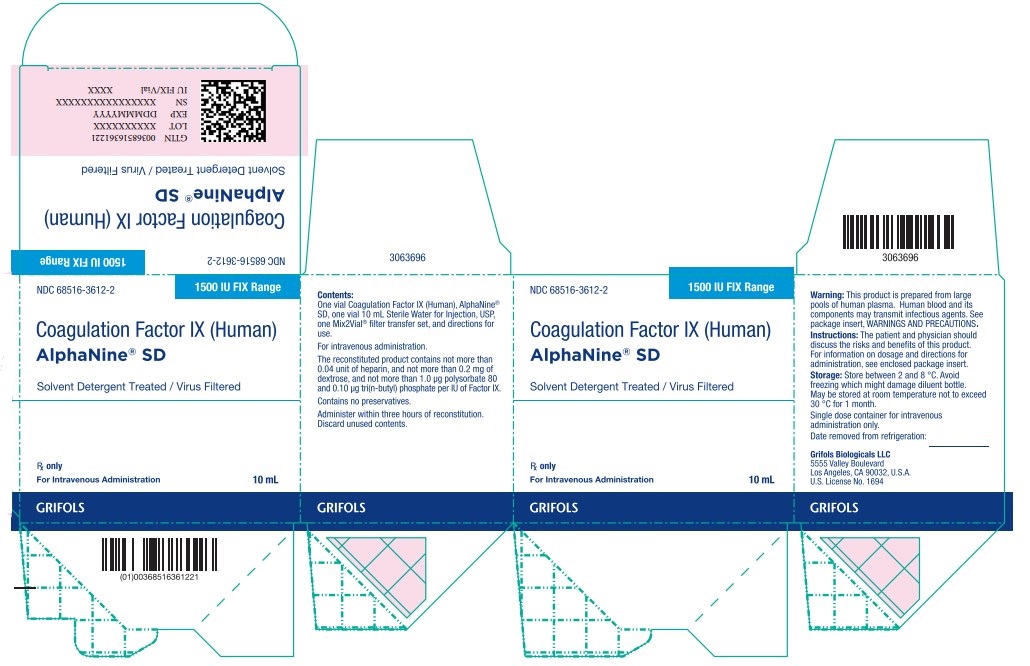
Principal Display Panel – 1500 IU Carton Label
NDC 68516-3612-2 1500 IU FIX Range
Coagulation Factor IX (Human)
AlphaNine® SD
Solvent Detergent Treated / Virus Filtered
Rx only
For Intravenous Administration 10 mL
GRIFOLS
Contents:
One vial Coagulation Factor IX (Human), AlphaNine®
SD,one vial 10 mL Sterile Water for Injection, USP,
one Mix2Vial® filter transfer set, and directions for
use.
For intravenous administration.
The reconstituted product contains not more than
0.04 unit of heparin, and not more than 0.2 mg of
dextrose, and not more than 1.0 µg polysorbate 80
and 0.10 µg tri(n-butyl) phosphate per IU of Factor IX.
Contains no preservatives.
Administer within three hours of reconstitution.
Discard unused contents.
GRIFOLS
Warning: This product is prepared from large
pools of human plasma. Human blood and its
components may transmit infectious agents. See
package insert, WARNINGS AND PRECAUTIONS.
Instructions: The patient and physician should
discuss the risks and benefits of this product.
For information on dosage and directions for
administration, see enclosed package insert.
Storage: Store between 2 and 8 °C. Avoid
freezing which might damage diluent bottle.
May be stored at room temperature not to exceed
30 °C for 1 month.
Single dose container for intravenous
administration only.
Date removed from refrigeration:
Grifols Biologicals LLC
5555 Valley Boulevard
Los Angeles, CA 90032, U.S.A.
U.S. License No 1694
GRIFOLS
GTIN 00368516361221
LOT XXXXXXXXXX
EXP DDMMMYYYY
SN XXXXXXXXXX XXXXXX
IU FI X/Vial XXXX
3063696
NDC 76297-002-12
Sterile Water for Injection, USP
10 mL
Rx Only
For reconstitution of accompanying product
Single-Dose Container, Nonpyrogenic
Do not use unless clear. No antimicrobial agent or other substance has been added. Do not use for intravascular injection without making approximately isotonic by addition of suitable solute. Discard unused portion.
Mfd by: Laboratorios Grifols, S. A. Parets del Vallès,
Barcelona 08150 Spain
Lot
EXP
3057423

NDC 68516-1002-2
Sterile Water for Injection, USP
10 mL
Rx Only
For reconstitution of accompanying product
Single-Dose Container, Nonpyrogenic
Do not use unless clear. No antimicrobial agent or other substance has been added. Do not use for intravascular injection without making approximately isotonic by addition of suitable solute. Discard unused portion.
Mfd by: Laboratorios Grifols, S. A. Parets del Vallès, Barcelona 08150 Spain
Mfd for: Grifols Biologicals LLC Los Angeles, CA 90032, USA
Lot
EXP
3051533