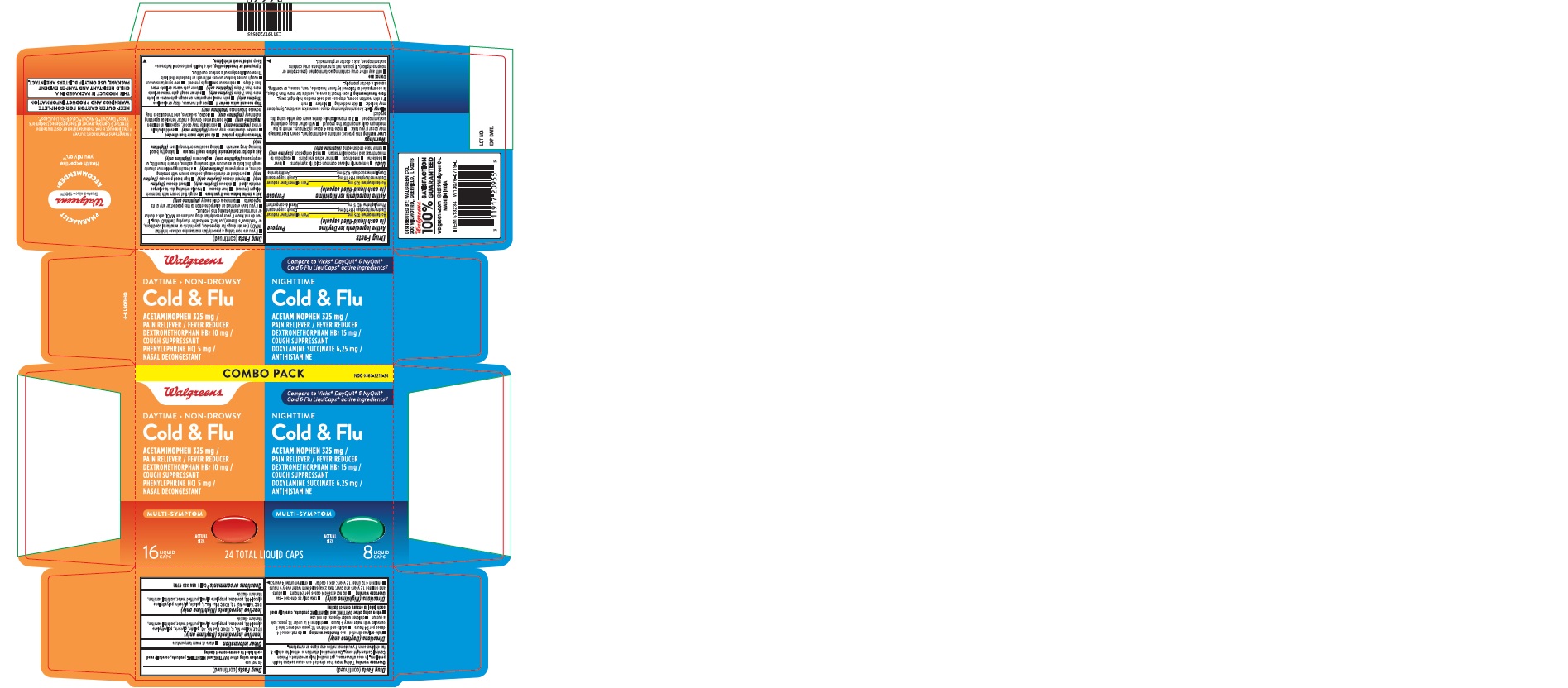
Active ingredients (in each liquid-filled capsule) (Daytime Cold & Flu)
Acetaminophen 325 mg
Dextromethorphan HBr 10 mg
Phenylephrine HCl 5 mg
Active ingredients (in each liquid-filled capsule) (Nighttime Cold & Flu)
Acetaminophen 325 mg
Dextromethorphan HBr 15 mg
Doxylamine succinate 6.25 mg
Uses
- temporarily relieves common cold and flu symptoms:
- fever
- headache
- sore throat
- minor aches and pains
- cough due to minor throat and bronchial irritation
- nasal congestion (Daytime only)
- runny nose and sneezing (Nighttime only)
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- if you have ever had an allergic reaction to this product or any of its ingredients
- to make a child sleepy (Nighttime only)
Ask a doctor before use if you have
- cough that occurs with too much phlegm (mucus)
- liver disease
- difficulty in urination due to enlargement of the prostate gland
- heart disease (Daytime only)
- diabetes (Daytime only)
- thyroid disease (Daytime only)
- high blood pressure (Daytime only)
- persistent or chronic cough such as occurs with smoking, asthma, or emphysema (Daytime only)
- a breathing problem or chronic cough that lasts or as occurs with smoking, asthma, chronic bronchitis, or emphysema (Nighttime only)
- glaucoma (Nighttime only)
Ask a doctor or pharmacist before use if you are
- taking the blood thinning drug warfarin
- taking sedatives or tranquilizers (Nighttime only)
When using this product
- do not exceed recommended dosage
- excitability may occur, especially in children (Nighttime only)
- avoid alcoholic beverages (Nighttime only)
- marked drowsiness may occur (Nighttime only)
- alcohol, sedatives, and tranquilizers may increase drowsiness (Nighttime only)
- use caution when driving a motor vehicle or operating machinery (Nighttime only)
Stop use and ask a doctor if
- nervousness, dizziness, or sleeplessness occur (Daytime only)
- pain, nasal congestion, or cough gets worse or lasts more than 7 days (Daytime only)
- pain or cough gets worse or lasts more than 7 days (Nighttime only)
- fever gets worse or lasts more than 3 days
- redness or swelling is present
- new symptoms occur
- cough comes back or occurs with rash or headache that lasts. These could be signs of a serious condition.
Keep out of reach of children.
In case of accidental overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away. Prompt medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
If taking NIGHTTIME and DAYTIME products, carefully read each section to ensure correct dosing.
Directions (Daytime only)
- do not take more than directed
- do not take more than 8 capsules per 24 hours
- adults and children 12 years and over: take 2 capsules with water every 4 hours
- children under 12 years: ask a doctor
Directions (Nighttime only)
-
do not take more than directed
- do not take more than 8 capsules per 24 hours
- adults and children 12 years and over: take 2 capsules with water every 6 hours
- children under 12 years: ask a doctor
Other information
- TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- protect from heat, humidity and light
- see end flap for expiration date and lot number
Inactive ingredients (Daytime only)
FD&C Yellow No. 6, FD&C Red No. 40, gelatin, glycerin, polyethylene
glycol 400, povidone, propylene glycol, purified water, sorbitol sorbitan,
titanium dioxide
Inactive ingredients (Nighttime only)
D&C Yellow No. 10, FD&C Blue No. 1, gelatin, glycerin, polyethylene
glycol 400, povidone, propylene glycol, purified water, sorbitol sorbitan,
titanium dioxide
Principal Display Panel
DAY & NIGHT PACK
Walgreens
Compare to Vicks® DayQuil® &
NyQuil® Cold & Flu LiquiCaps®
active ingredients
††
|
DAYTIME • NON DROWSY
ACETAMINOPHEN /
MULTI-SYMPTOM ACTUAL
16
|
NIGHTTIME
ACETAMINOPHEN /
MULTI-SYMPTOM ACTUAL
8
|
24 TOTAL LIQUID CAPS
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED
OR IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY
SIGNS OF TAMPERING
Walgreens Pharmacist Recommended
Walgreens Pharmacist Survey
††This product is not manufactured or
distributed by Procter & Gamble, owner of the
registered trademark Vicks® DayQuil® &
NyQuil® Cold & Flu LiquiCaps®.
DISTRIBUTED BY: WALGREEN CO.
200 WILMOT RD., DEERFIELD, IL 60015