Warnings
For external use only.
When using this product
■ skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne treatment at a time. ■ avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
Stop use and ask a doctor if
■ irritation becomes severe
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
•Sensitivity Test for New User: Apply product sparingly to one or two small affected areas during the first 3 days. If no discomfort occurs, follow directions stated below. • clean the skin thoroughly before applying this product. • cover the entire affected area with a thin layer one to three times daily • because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor. • if bothersome dryness or peeling occurs, reduce application to once a day or every other day.
Inactive Ingredients
WATER, GLYCERIN, COCO-CAPRYLATE/CAPRATE, GLUCONOLACTONE, PROPANEDIOL, SODIUM HYDROXIDE, SUCCINIC ACID, PANTHENOL, NIACINAMIDE, LYSOLECITHIN, SCLEROTIUM GUM, ZINC GLUCONATE, PULLULAN, XANTHAN GUM, PANTOLACTONE, CITRIC ACID, SILICA
Distributed by:
Galderma Laboratories, L.P.
Dallas, TX 75201 USA
All trademarks are the property of their respective owners.
cetaphil.com.
P56577-1
Principle Display Panel - 1 FL OZ Carton
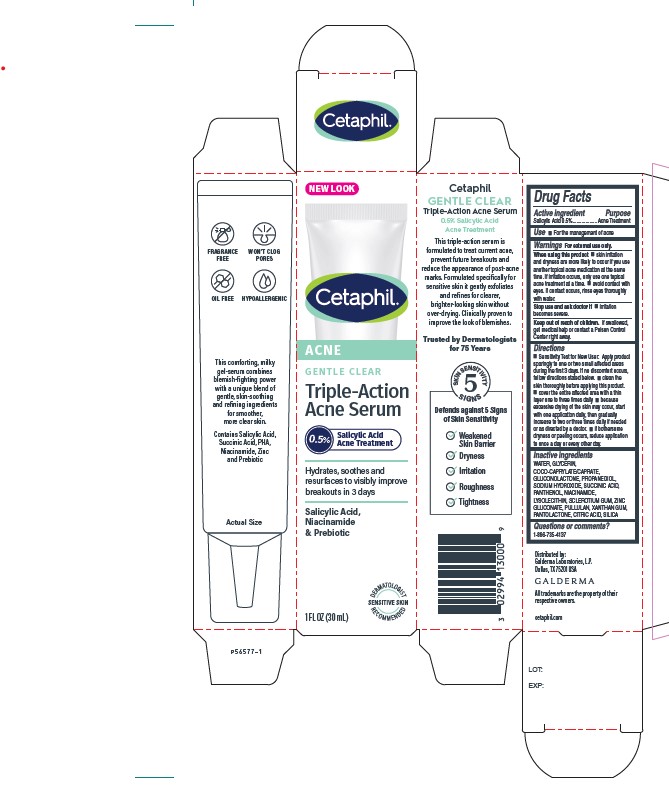
NEW LOOK
Cetaphil®
ACNE
GENTLE CLEAR
Triple-Action
Acne Serum
0.5% Salicylic Acid Acne Treatment
Hydrates, soothes and
resurfaces to visibly improve
breakouts in 3 days
Salicylic Acid,
Niacinamide
& Prebiotic
Dermatologist Recommended
Sensitive Skin
1 FL OZ (30 mL)