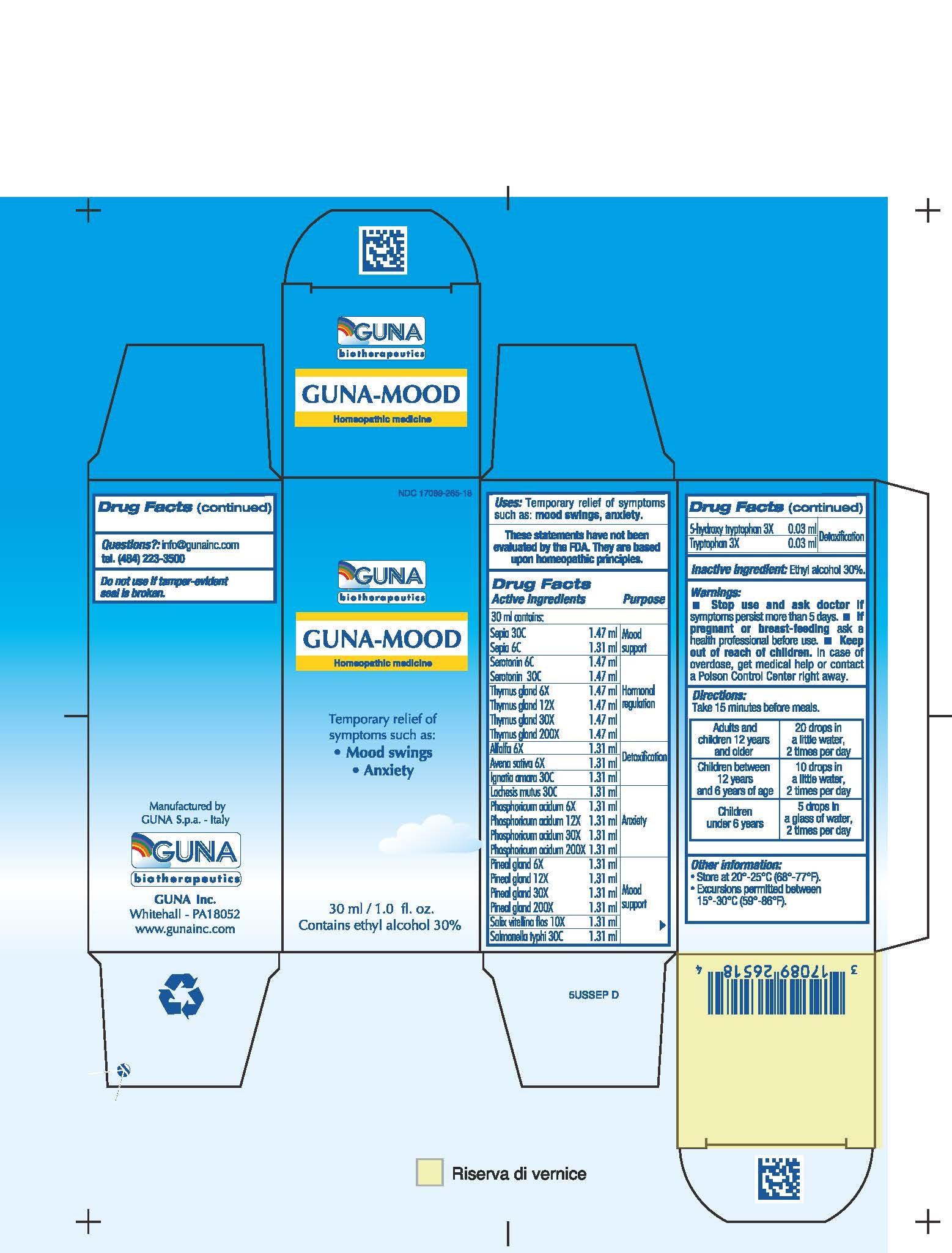
ACTIVE INGREDIENTS/PURPOSE
5-HYDROXY TRYPTOPHAN 3X DETOXIFICATION
ALFALFA 6X DETOXIFICATION
AVENA SATIVA 6X DETOXIFICATION
IGNATIA AMARA 30C ANXIETY
LACHESIS MUTUS 30C ANXIETY
PHOSPHORICUM ACIDUM 6X 12X 30X 200X ANXIETY
PINEAL GLAND 6X 12X 30X 200X HORMONAL REGULATION
SALIX VITELLINA FLOS 10X MOOD SUPPORT
SALMONELLA TYPHI 30C MOOD SUPPORT
SEPIA 6C 30C MOOD SUPPORT
SEROTONIN 6C 30C HORMONAL REGULATION
THYMUS GLAND 6X 12X 30X 200X HORMONAL REGULATION
TRYPTOPHAN 3X DETOXIFICATION
WARNINGS
- Stop use and ask doctor if symptoms persist more than 5 days.
- If pregnant or breast-feeding ask a health professional before use.
- Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
- Contains ethyl alcohol 30%