Warnings
For external use only
Do not use
- more than 1 patch on your body at a time or on cut, irritated or swollen skin
- on puncture wounds
- for more than one week without consulting a doctor
When using this product
- use only as directed. Read and follow all directions and warnings on this carton.
- do not allow contacts with the eyes
- do not bandage tightly or apply local heat (such as heating pads) to the area of use
- do not use at the same time as other topical analgesics
- dispose of used patch in manner that always keeps product away from children and pets. Used patches still contain the drug product that can produce serious adverse effects if a child or pet chews or ingests this patch.
Directions
adults and children over 12 years:
- clean and dry affected area
- remove film from patch
- carefully apply patch to affected area
- use 1 patch for up to 12 hours
children 12 years or younger: ask a doctor
Inactive ingredients
polyvinyl alcohol, non-crystalizing sorbitol solution, polyacrylic acid, glycerin, carboxymethylcellulose sodium, colloidal silicon dioxide, titanium dioxide, propylene glycol, tartaric acid, magnesium hydroxide, sodium polyacrylate, purified water
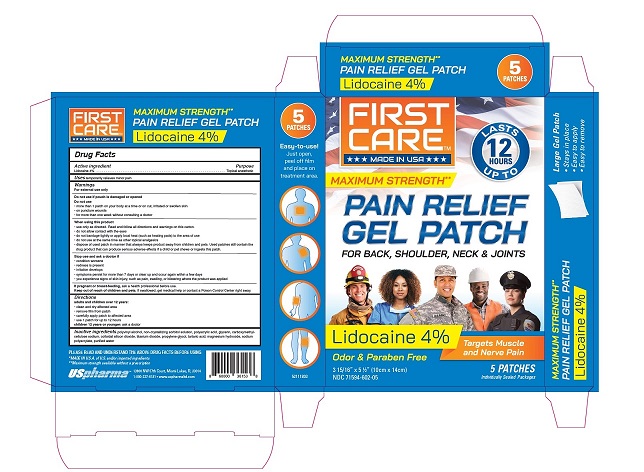
Package/Label Principal Display Panel
FIRST CARE TM
***MADE IN USA***
LASTS UP TO 12 HOURS
MAXIMUM STRENGTH**
PAIN RELIEF GEL PATCH
FOR BACK, SHOULDER, NECK & JOINTS
Lidocaine 4%
Targets Muscle and Nerve Pain
Odor & Paraben Free
3-15/16” x 5-1/2” (10 cm x 14 cm)
NDC 71594-602-05
5 PATCHES
Individually sealed in child resistant packages