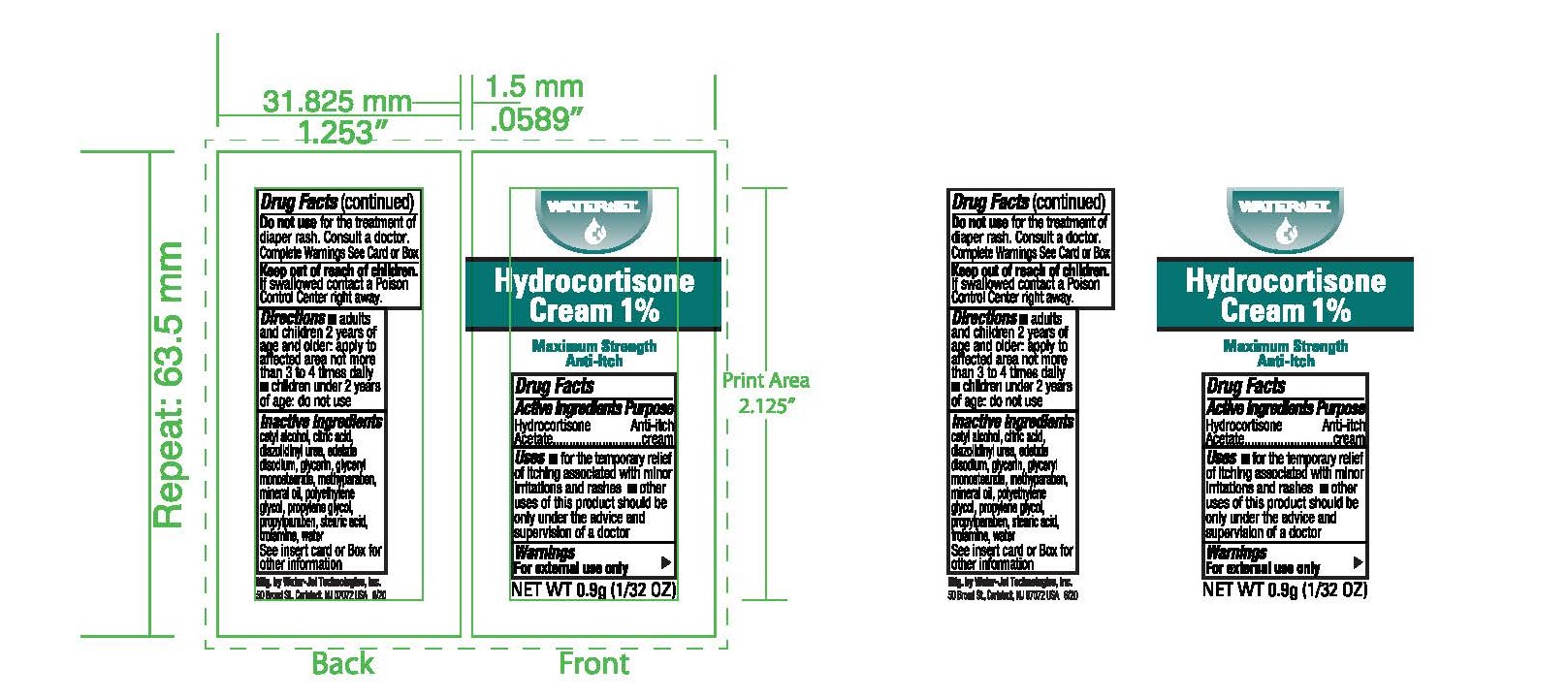
Uses
- for the temporary relief of itching associated with minor skin irritations and rashes
- other uses of this product should be only under the advice and supervision of a doctor
Warnings
For external use only
When using this product
- avoid contact with eyes
- do not begin use of any other hydrocortisone product unless you've consulted a doctor
Directions
- adults and children 2 years of age and older: apply to affected area not more than 3 to 4 times daily
- children under 2 years; do not use, consult a doctor
Other information
- clean the affected area
- store at room temperature
- do not use any opened or torn packets
- you may report a serious adverse reaction to this product to 800-275-3433