DESCRIPTION
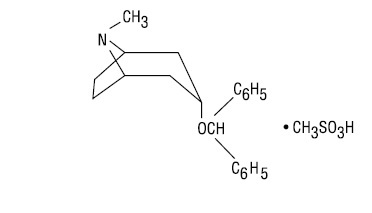
Benztropine mesylate, USP is a synthetic compound containing structural features found in atropine and diphenhydramine.
It is a crystalline white powder, very soluble in water, designated as 3α-(Diphenylmethoxy)-1αH, 5αH-tropane methanesulfonate, with the following structural formula:
Each tablet, for oral administration, contains 0.5 mg, 1 mg or 2 mg of benztropine mesylate USP.
Each tablet contains the following inactive ingredients: lactose anhydrous, micro-crystalline cellulose, colloidal silicon dioxide, sodium starch glycolate and magnesium stearate.
CLINICAL PHARMACOLOGY
Benztropine mesylate possesses both anticholinergic and antihistaminic effects, although only the former have been established as therapeutically significant in the management of parkinsonism.
In the isolated guinea pig ileum, the anticholinergic activity of this drug is about equal to that of atropine; however, when administered orally to unanesthetized cats, it is only about half as active as atropine.
In laboratory animals, its antihistaminic activity and duration of action approach those of pyrilamine maleate.
INDICATIONS AND USAGE
For use as an adjunct in the therapy of all forms of parkinsonism.
Useful also in the control of extrapyramidal disorders (except tardive dyskinesia – see PRECAUTIONS) due to neuroleptic drugs (e.g., phenothiazines).
CONTRAINDICATIONS
Hypersensitivity to benztropine mesylate tablets.
Because of its atropine-like side effects, this drug is contraindicated in pediatric patients under three years of age, and should be used with caution in older pediatric patients.
WARNINGS
Safe use in pregnancy has not been established.
Benztropine mesylate may impair mental and/or physical abilities required for performance of hazardous tasks, such as operating machinery or driving a motor vehicle.
When benztropine mesylate is given concomitantly with phenothiazines, haloperidol, or other drugs with anticholinergic or antidopaminergic activity, patients should be advised to report gastrointestinal complaints, fever or heat intolerance promptly. Paralytic ileus, hyperthermia and heat stroke, all of which have sometimes been fatal, have occurred in patients taking anticholinergic-type antiparkinsonism drugs, including benztropine mesylate, in combination with phenothiazines and/or tricyclic antidepressants.
Since benztropine mesylate contains structural features of atropine, it may produce anhidrosis. For this reason, it should be administered with caution during hot weather, especially when given concomitantly with other atropine-like drugs to the chronically ill, the alcoholic, those who have central nervous system disease, and those who do manual labor in a hot environment. Anhidrosis may occur more readily when some disturbance of sweating already exists. If there is evidence of anhidrosis, the possibility of hyperthermia should be considered. Dosage should be decreased at the discretion of the physician so that the ability to maintain body heat equilibrium by perspiration is not impaired. Severe anhidrosis and fatal hyperthermia have occurred.
PRECAUTIONS
General
Since benztropine mesylate has cumulative action, continued supervision is advisable. Patients with a tendency to tachycardia and patients with prostatic hypertrophy should be observed closely during treatment.
Dysuria may occur, but rarely becomes a problem. Urinary retention has been reported with benztropine mesylate.
The drug may cause complaints of weakness and inability to move particular muscle groups, especially in large doses. For example, if the neck has been rigid and suddenly relaxes, it may feel weak, causing some concern. In this event, dosage adjustment is required.
Mental confusion and excitement may occur with large doses, or in susceptible patients. Visual hallucinations have been reported occasionally. Furthermore, in the treatment of extrapyramidal disorders due to neuroleptic drugs (e.g., phenothiazines), in patients with mental disorders, occasionally there may be intensification of mental symptoms. In such cases, antiparkinsonian drugs can precipitate a toxic psychosis. Patients with mental disorders should be kept under careful observation, especially at the beginning of treatment or if dosage is increased.
Tardive dyskinesia may appear in some patients on long-term therapy with phenothiazines and related agents, or may occur after therapy with these drugs has been discontinued. Antiparkinsonism agents do not alleviate the symptoms of tardive dyskinesia, and in some instances may aggravate them. Benztropine mesylate is not recommended for use in patients with tardive dyskinesia.
The physician should be aware of the possible occurrence of glaucoma. Although the drug does not appear to have any adverse effect on simple glaucoma, it probably should not be used in
angle-closure glaucoma.
Drug Interactions
Antipsychotic drugs such as phenothiazines or haloperidol; tricyclic antidepressants (see WARNINGS).
Pediatric Use
Because of the atropine-like side effects, benztropine mesylate should be used with caution in pediatric patients over three years of age (see CONTRAINDICATIONS).
ADVERSE REACTIONS
The adverse reactions below, most of which are antichlolinergic in nature, have been reported and within each category are listed in order of decreasing severity.
Cardiovascular
Tachycardia.
Digestive
Paralytic ileus, constipation, vomiting, nausea, dry mouth.
If dry mouth is so severe that there is difficulty in swallowing or speaking, or loss of appetite and weight, reduce dosage, or discontinue the drug temporarily.
Slight reduction in dosage may control nausea and still give sufficient relief of symptoms. Vomiting may be controlled by temporary discontinuation, followed by resumption at a lower dosage.
Nervous System
Toxic psychosis, including confusion, disorientation, memory impairment, visual hallucinations; exacerbation of pre-existing psychotic symptoms; nervousness; depression; listlessness; numbness of fingers.
Special Senses
Blurred vision, dilated pupils.
Urogenital
Urinary retention, dysuria.
Metabolic/Immune or Skin
Occasionally, an allergic reaction, e.g., skin rash, develops. If this can not be controlled by dosage reduction, the medication should be discontinued.
Other
Heat stroke, hyperthermia, fever.
OVERDOSAGE
Manifestations – May be any of those seen in atropine poisoning or antihistamine overdosage: CNS depression, preceded or followed by stimulation; confusion; nervousness; listlessness; intensification of mental symptoms or toxic psychosis in patients with mental illness being treated with neuroleptic drugs (e.g., phenothiazines); hallucinations (especially visual); dizziness; muscle weakness; ataxia; dry mouth; mydriasis; blurred vision; palpitations; tachycardia; elevated blood pressure; nausea; vomiting; dysuria; numbness of fingers; dysphagia; allergic reactions, e.g., skin rash; headache; hot, dry, flushed skin; delirium; coma; shock; convulsions; respiratory arrest; anhidrosis; hyperthermia; glaucoma; constipation.
Treatment – Physostigmine salicylate, 1 to 2 mg, SC or IV, reportedly will reverse symptoms of anticholinergic intoxication. * A second injection may be given after 2 hours if required. Otherwise treatment is symptomatic and supportive. Induce emesis or perform gastric lavage (contraindicated in precomatose convulsive, or psychotic states). Maintain respiration. A short-acting barbiturate may be used for CNS excitement, but with caution to avoid subsequent depression; supportive care for depression (avoid convulsant stimulants such as picrotoxin, pentylenetetrazol, or bemegride); artificial respiration for severe respiratory depression; a local miotic for mydriasis and cycloplegia; ice bags or other cold applications and alcohol sponges for hyperpyrexia, a vasopressor and fluids for circulatory collapse. Darken room for photophobia.
DOSAGE AND ADMINISTRATION
Benztropine mesylate tablets should be used when patients are able to take oral medication.
Because of cumulative action, therapy should be initiated with a low dose which is increased gradually at five or six-day intervals to the smallest amount necessary for optimal relief. Increases should be made in increments of 0.5 mg, to a maximum of 6 mg, or until optimal results are obtained without excessive adverse reactions.
Postencephalitic and Idiopathic Parkinsonism – The usual daily dose is 1 to 2 mg, with a range of 0.5 to 6 mg orally.
As with any agent used in parkinsonism, dosage must be individualized according to age and weight, and the type of parkinsonism being treated. Generally, older patients, and thin patients cannot tolerate large doses. Most patients with postencephalitic parkinsonism need fairly large doses and tolerate them well. Patients with a poor mental outlook are usually poor candidates for therapy.
In idiopathic parkinsonism, therapy may be initiated with a single daily dose of 0.5 to 1 mg at bedtime. In some patients, this will be adequate; in others 4 to 6 mg a day may be required.
In postencephalitic parkinsonism, therapy may be initiated in most patients with 2 mg a day in one or more doses. In highly sensitive patients, therapy may be initiated with 0.5 mg at bedtime, and increased as necessary.
Some patients experience greatest relief by taking the entire dose at bedtime; others react more favorably to divided doses, two to four times a day. Frequently, one dose a day is sufficient, and divided doses may be unnecessary or undesirable.
The long duration of action of this drug makes it particularly suitable for bedtime medication when its effects may last throughout the night, enabling patients to turn in bed during the night more easily, and to rise in the morning.
When benztropine mesylate is started, do not terminate therapy with other antiparkinsonian agents abruptly. If the other agents are to be reduced or discontinued, it must be done gradually. Many patients obtain greatest relief with combination therapy.
Benztropine mesylate may be used concomitantly with Carbidopa- Levodopa, or with levodopa, in which case periodic dosage adjustment may be required in order to maintain optimum response.
Drug-Induced Extrapyramidal Disorders – In treating extrapyramidal disorders due to neuroleptic drugs (e.g., phenothiazines), the recommended dosage is 1 to 4 mg once or twice a day orally. Dosage must be individualized according to the need of the patient. Some patients require more than recommended; others do not need as much.
When extrapyramidal disorders develop soon after initiation of treatment with neuroleptic drugs (e.g., phenothiazines), they are likely to be transient. One to 2 mg of benztropine mesylate tablets two or three times a day usually provides relief within one or two days. After one or two weeks the drug should be withdrawn to determine the continued need for it. If such disorders recur, benztropine mesylate can be reinstituted.
Certain drug-induced extrapyramidal disorders that develop slowly may not respond to benztropine mesylate.
HOW SUPPLIED
Benztropine Mesylate Tablets, USP are available as follows:
0.5 mg white, capsule shaped biconvex tablets de-bossed with 'I' on the left side of bisect and 'G'on the right side of the bisect and "318" on the other side, supplied in bottles of 30 (NDC 69097-826-02), 100 (NDC 69097-826-07) and 1000 (NDC 69097-826-15).
1 mg white, modified oval biconvex tablets de-bossed with "I" on the left side of bisect and "G" on the right side of the bisect on one side and "319" on the other side, supplied in bottles of 30 (NDC 69097-827-02), 100 (NDC 69097-827-07) and 1000 (NDC 69097-827-15).
2 mg white, round, flat faced beveled edged tablets de-bossed with 'I' on the left side of bisect and 'G' on the right side of the bisect and "320" on the other side, supplied in bottles of 30 (NDC 69097-832-02), 100 (NDC 69097-832-07) and 1000 (NDC 69097-832-15).
Dispense in a well-closed container as defined in the USP.
Store at 20° to 25°C (68° to77°F) [See USP Controlled Room Temperature].
*Duvoisin, R.C.; Katz, R.J.; Amer. Med.Ass. 206:1963-1965, Nov. 25, 1968.
Manufactured by:
InvaGen Pharmaceuticals, Inc.
(a subsidiary of Cipla Ltd.)
Hauppauge, NY 11788
Manufactured for:
Cipla USA, Inc.
10 Independence Boulevard, Suite 300 Warren, NJ 07059
Revised: 02/2020