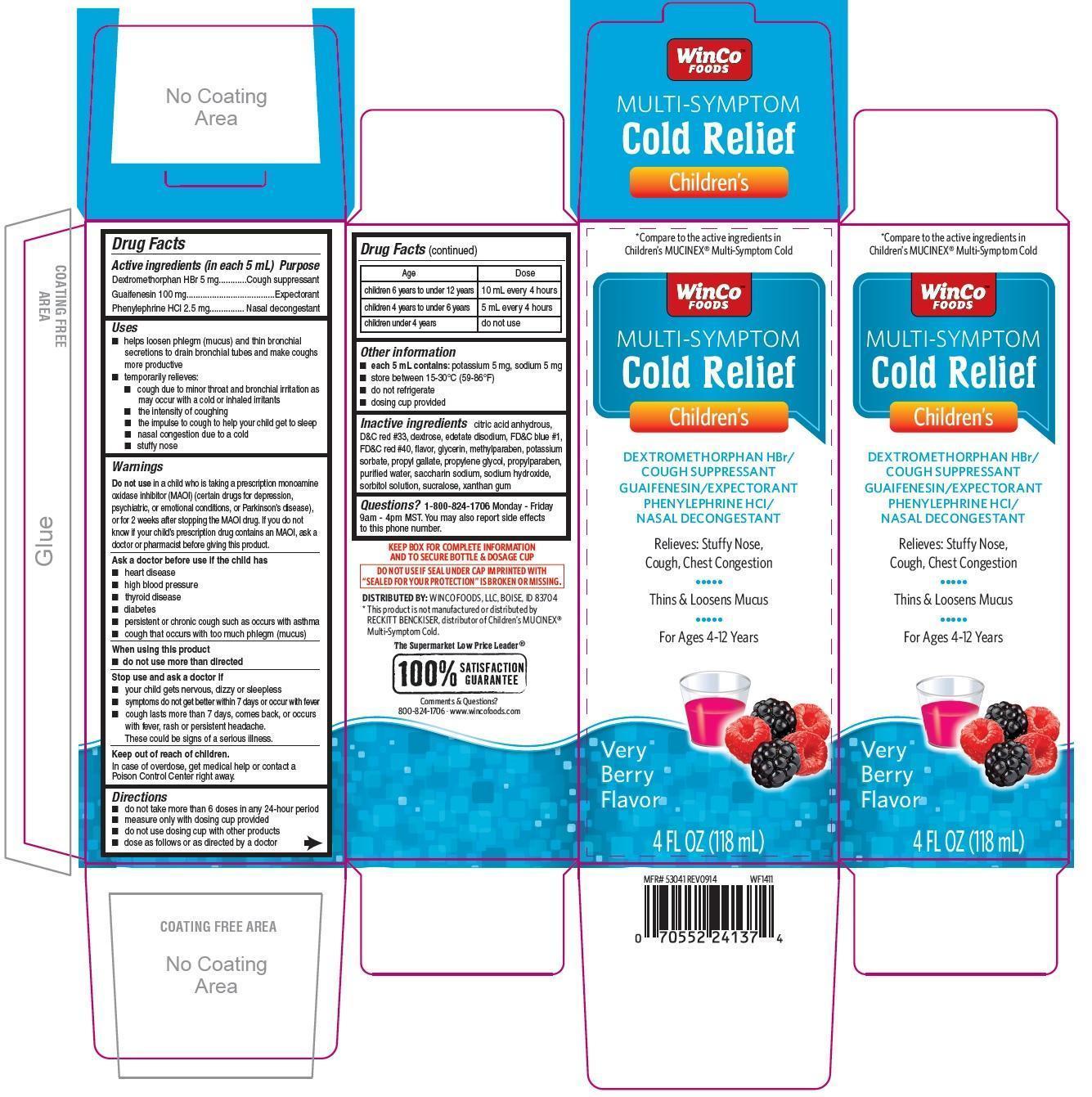
Active ingredients (in each 5 mL)
Dextromethorphan HBr 5 mg
Guaifenesin 100 mg
Phenylephrine HCl 2.5 mg
Uses
- helps loosen phlegm (mucus) and thin bronchial secretions to drain bronchial tubes and make coughs more productive
- temporarily relieves:
- cough due to minor throat and bronchial irritation as may occur with a cold or inhaled irritants
- the intensity of coughing
- the impulse to cough to help your child get to sleep
- nasal congestion due to a cold
- stuffy nose
Warnings
Do not use in a child who is taking a prescription monoamine oxidase inhibitor (MAOI)(certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before giving this product.
Ask a doctor before use if the child has
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- persistent or chronic cough such as occurs with asthma
- cough that occurs with too much phlegm (mucus)
When using this product
- do not use more than directed
Stop use and ask a doctor if
- your child gets nervous, dizzy or sleepless
- symptoms do not get better within 7 days or occur with fever
- cough lasts more than 7 days, comes back, or occurs with fever, rash or persistent headache. These could be signs of a serious illness.
keep out of reach of children. In case of overdose, get medical help or contact a poison control center right away.
Directions
- do not take more than 6 doses in any 24 hour period
- measure only with dosing cup provided
- do not use dosing cup with other products
- dose as follows or as directed by a doctor
| Age | Dose |
| children 6 years to under 12 years | 10 mL every 4 hours |
| children 4 years to under 6 years | 5 mL every 4 hours |
| children under 4 years | do not use |
Other information
- each 5 mL contains: potassium 5 mg, sodium 5 mg
- store between 15-30oC (59-86oF)
- do not refrigerate
- dosing cup provided
Inactive ingredients
citric acid anhydrous, D&C red 33, dextrose, edetate disodium, FD&C blue 1, FD&C red 40, flavor, glycerin, methylparaben, potassium sorbate, propyl gallate, propylene glycol, propylparaben, purified water, saccharin sodium, sodium hydroxide, sorbitol solution, sucralose, xanthan gum
Questions?
1-800-824-1706 Monday - Friday 9am-4pm MST.
You may also report side effects to this phone number
PDP
*Compare to the active ingredients in Children's MUCINEX® Multi-symptom cold
Children's Multi-symptom Cold Relief
Dextromethorphan HBr / Cough suppressant
Guaifenesin / Expectorant
Phenylephrine HCl / Nasal decongestant
Relieves:Stuffy Nose, Cough, Chest Congestion
Thins and Loosens Mucus
For Ages 4-12 Years
Ver Berry Flavor
4 FL OZ (118 mL)