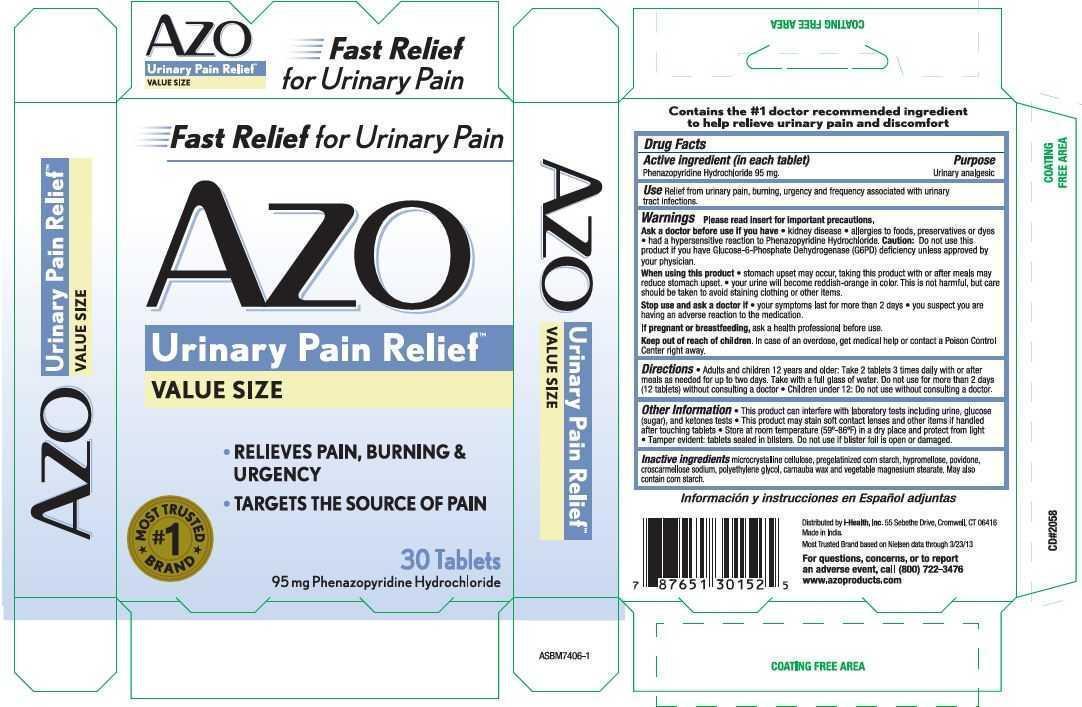
Use Relief from urinary pain, burning, urgency and frequency associated with urinary tract infections.
Warnings Please read insert for important precautions.
Ask a doctor before use if you have
- kidney disease
- allergies to foods, preservatives or dyes
- had a hypersensitive reaction to Phenazopyridine Hydrochloride.
Caution: Do not use this product if you have Glucose-6-Phosphate Dehydrogenase (G6PD) deficiency unless approved by your physician.
Keep out of reach of children. In case of an overdose, get medical help or contact a Poison Control Center right away.
Directions
- Adults and children 12 years or older: Take 2 tablets 3 times daily with or after meals as needed for up to two days. Take with a full glass of water. Do not use for more than 2 days (12 tablets) without consulting a doctor.
- Children under 12: Do not use without consulting a doctor.
Other Information
- This product can interfere with laboratory tests including urine, glucose (sugar), and ketones tests
- This product may stain soft contact lenses and other items if handled after touching tablets
- Store at room temperature (59-86 F) in a dry place and protect from light
- Tamper evident: tablets sealed in blisters. Do not use if blister foil or seal is open or damaged.
Inactive ingredientsmicrocrystalline cellulose, pregelatinized corn starch, hypromellose, povidine, croscarmellose sodium, polyethylene glycol, carnauba wax and vegetable magnesium stearate. May also contain corn starch.