KETOTIFEN FUMARATE- ketotifen fumarate solution
CVS Pharmacy
----------
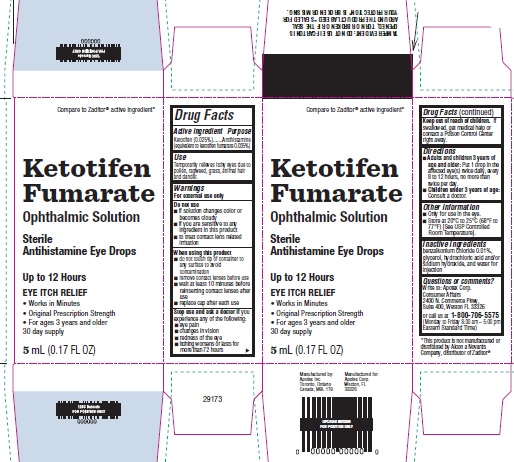
Drug Facts
Warnings
For external use only
Do not use
- if solution changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
- to treat contact lens related irritation
When using this product
- do not touch tip of container to any surface to avoid contamination
- remove contact lenses before use
- wait at least 10 minutes before reinserting contact lenses after use
- replace cap after each use
Directions
- Adults and children 3 years of age and older: Put 1 drop in the affected eye(s) twice daily, every 8 to 12 hours, no more than twice per day.
- Children under 3 years of age: Consult a doctor.
Other information
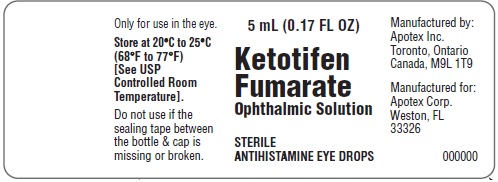
- Only for use in the eye.
- Store at 20°C to 25°C (68°F to 77°F) [See USP Controlled Room Temperature].
Inactive ingredients
benzalkonium chloride 0.01%, glycerol, hydrochloric acid and/or sodium hydroxide, and water for injection
Questions or comments?
Write to:
Apotex Corp.
Consumer Affairs
2400 N. Commerce Pkwy,
Suite 400,
Weston FL 33326
or call us at 1-800-706-5575 (Monday to Friday 8:30 am – 5:00 pm Eastern Standard Time)
| KETOTIFEN FUMARATE
ketotifen fumarate solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - CVS Pharmacy (062312574) |
| Registrant - Apotex Inc (209429182) |
Revised: 10/2020
Document Id: 35f7245d-f96f-f431-5160-64ac93a5e87c
Set id: 3cd76a82-1cdb-d701-cf95-ef5b6ad1fc4c
Version: 2
Effective Time: 20201013
CVS Pharmacy