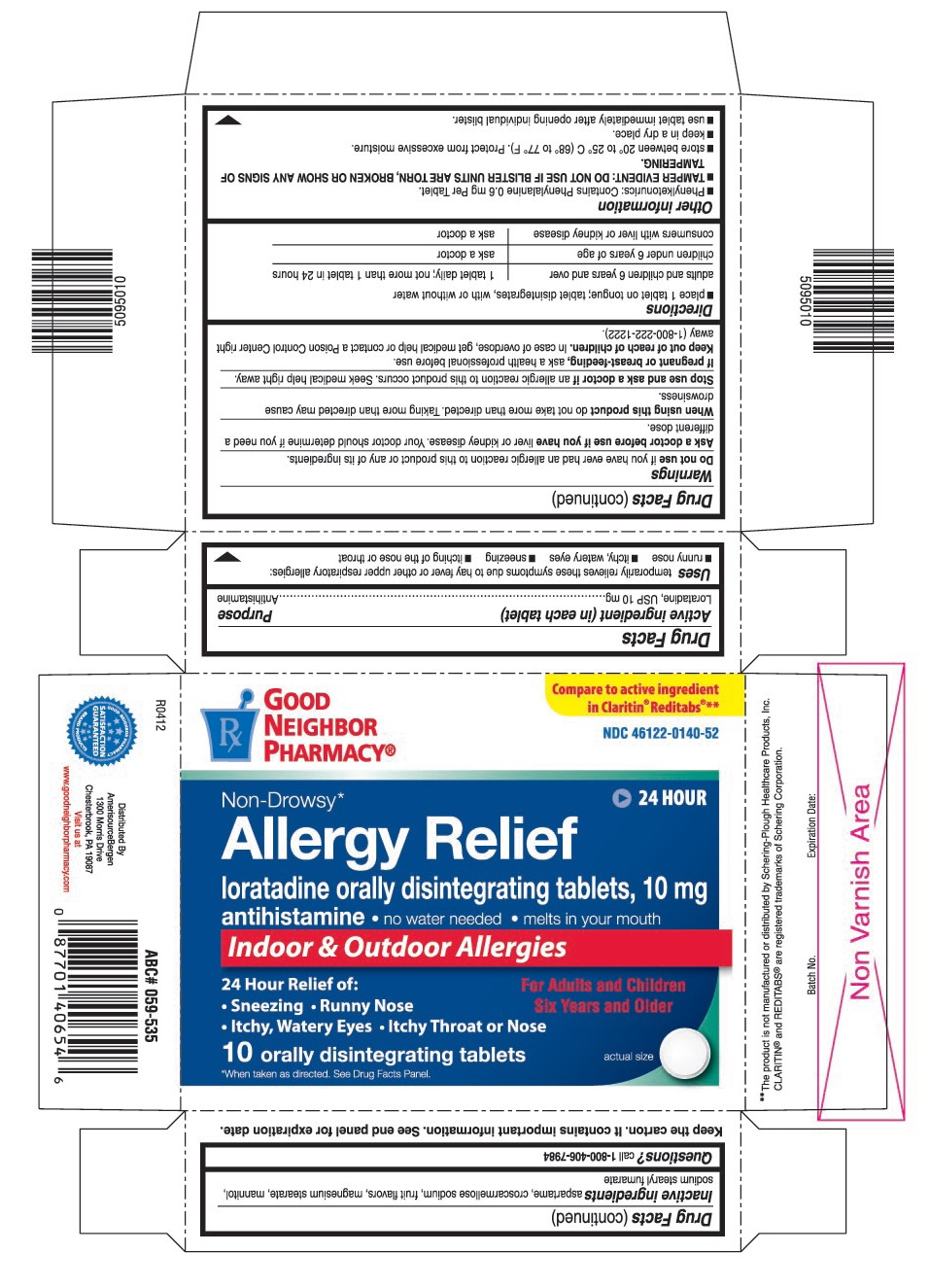
USES
Temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- itchy, watery eyes
- sneezing
- itching of the nose or throat
WARNINGS
Ask a doctor before use if you have
Liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
Do not take more than directed. Taking more than directed may cause drowsiness.
OTHER INFORMATION
- Phenylketonurics: Contains Phenylalanine 0.6 mg Per Tablet.
- TAMPER EVIDENT: DO NOT USE IF BLISTER UNITS ARE TORN, BROKEN OR SHOW ANY SIGNS OF TAMPERING.
- store between 20° to 25° C (68° to 77° F). Protect from excessive moisture.
- keep in a dry place.
- use tablet immediately after opening individual blister.
INACTIVE INGREDIENTS
Aspartame, croscarmellose sodium, fruit flavors, magnesium stearate, mannitol, sodium stearyl fumarate
PRINCIPAL DISPLAY PANEL
Compare to active ingredient in Claritin®Reditabs®**
loratadine orally disintegrating tablets, 10 mg
- no water needed
- melts in your mouth
- Sneezing
- Runny Nose
- Itchy, Watery Eyes
- Itchy Throat or Nose
For Adults and Children Six Years and Older
10 orally disintegrating tablets
*When taken as directed. See Drug Facts Panel.