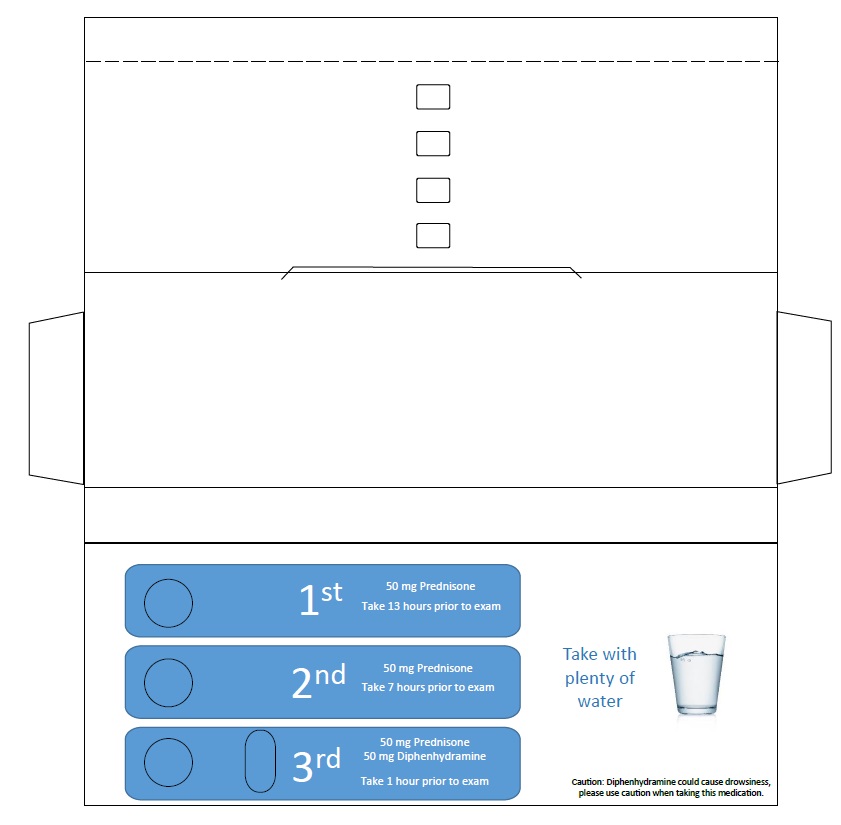
CONTRAST ALLERGY PREMED PACK- prednisone, diphenhydramine
Shertech Laboratories, LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Contrast Allergy PreMed Pack
| CONTRAST ALLERGY PREMED PACK
prednisone, diphenhydramine kit |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Shertech Laboratories, LLC (621117279) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Shertech Laboratories, LLC | 621117279 | manufacture(16129-101) | |
Revised: 12/2023
Document Id: 21e998d2-bb8a-4282-a11f-d4801d23bc76
Set id: 3c675b3b-3bd7-4219-e054-00144ff88e88
Version: 5
Effective Time: 20231227
Shertech Laboratories, LLC