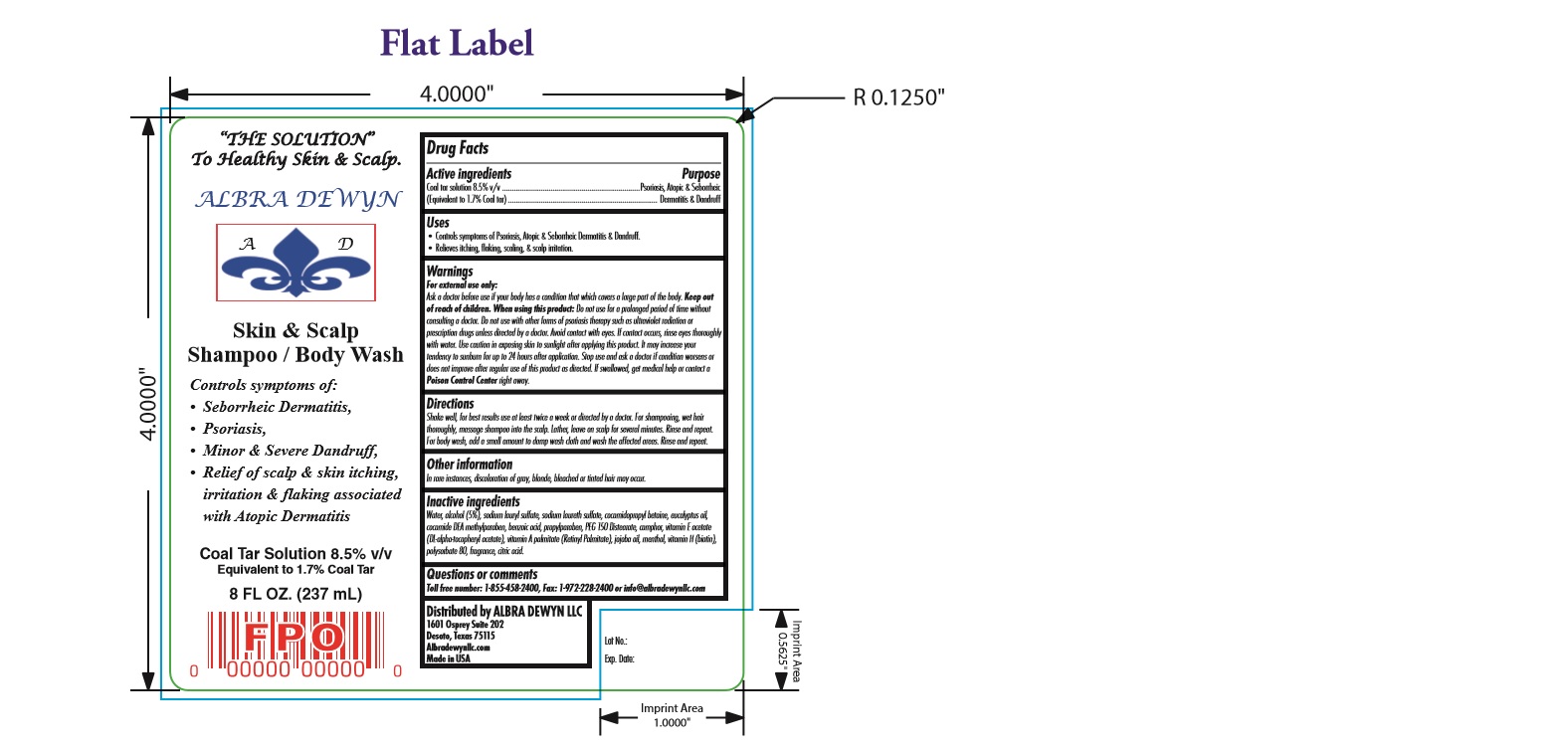
DRUG FACTS
Active ingredients Purpose
Coal tar solution 8.5% v/v .......................................................................Psoriasis, Atopic & Seborrheic
(Equivalent to 1.7% Coal tar)............................................................................. Dermatitis & Dandruff
USES
Controls symptoms of Psoriasis, Atopic & Seborrheic Dermatitis & Dandruff. • Relieves itching, flaking, scaling, & scalp irritation.
WARNINGS
For external use only: Ask a doctor before use if your body has a condition that which covers a large part of the body. Keep out of reach of children. When using this product: Do not use for a prolonged period of time without consulting a doctor. Do not use with other forms of psoriasis therapy such as ultraviolet radiation or prescription drugs unless directed by a doctor. Avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water. Use caution in exposing skin to sunlight after applying this product. It may increase your tendency to sunburn for up to 24 hours after application. Stop use and ask a doctor if condition worsens or does not improve after regular use of this product as directed. If swallowed, get medical help or contact a Poison Control Center right away.
DIRECTIONS
Shake well, for best results use at least twice a week or directed by a doctor. For shampooing, wet hair thoroughly, massage shampoo into the scalp. Lather, leave on scalp for several minutes. Rinse and repeat. For body wash, add a small amount to damp wash cloth and wash the affected areas. Rinse and repeat
Controls symptoms of: • Seborrheic Dermatitis, • Psoriasis, • Minor & Severe Dandruff, • Relief of scalp & skin itching, irritation & flaking associated with Atopic Dermatitis
OTHER INFORMATION
In rare instances, discoloration of gray, blonde, bleached or tinted hair may occur.
INactive
Inactive ingredients Water, alcohol (5%), sodium lauryl sulfate, sodium laureth sulfate, cocamidopropyl betaine, eucalyptus oil,
cocamide DEA methylparaben, benzoic acid, propylparaben, PEG 150 Distearate, camphor, vitamin E acetate (DL-alpha-tocopheryl acetate),
vitamin A palmitate (Retinyl Palmitate), jojoba oil, menthol, vitamin H (biotin), polysorbate 80, fragrance, citric acid.
questions or comments
Questions or comments Toll free number: 1-855-458-2400, Fax: 1-972-228-2400 or info@albradewynllc.com