Use(s)
• Helps prevent sunburn
• If used as directed with other sun protection measures (see Directions) decreases the risk of skin cancer and early skin aging caused by the sun.
Warnings
For external use only
Do not use on damaged or broken skin.
Stop use and ask a doctor if rash occurs.
When using this product, keep out of eyes.
Rinse with water to remove.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
• Apply liberally 15 minutes before sun exposure.
• Use a water resistant sunscreen if swimming or sweating
• Reapply at least every 2 hours.
• Children under 6 months: ask a doctor
SUN PROTECTION MEASURES: Spending time in the sun increases your risk of cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
• Limit time in the sun, especially 10am – 2 pm.
• Wear long-sleeved shirts, pants, hats, and sun glasses.
Inactive Ingredients
ALLANTOIN, BENZYL ALCOHOL, C12-15 ALKYL BENZOATE, CAFFEINE, CAPRYLYL METHICONE, CETYL DIGLYCERYL TRIS(TRIMETHYLSILOXY) SILYLETHYL DIMETHICONE, CHROMIUM OXIDE GREENS, DICAPRYLYL CARBONATE, DIMETHICONE, DIPOTASSIUM GLYCYRRHIZATE, ETHYLHEXYLGLYCERIN, GLYCERIN, HYDROGENATED LECITHIN, IRON OXIDES, ISOHEXADECANE, MAGNESIUM SULFAGE, MEHYLPROPANEDIOL, SILICA, TOCOPHEROL, TRIETHOXYCAPRYLYLSILANE, TRISILOXANE, WATER
PRINCIPAL DISPLAY PANEL - 1.7 FL OZ (50 mL) carton
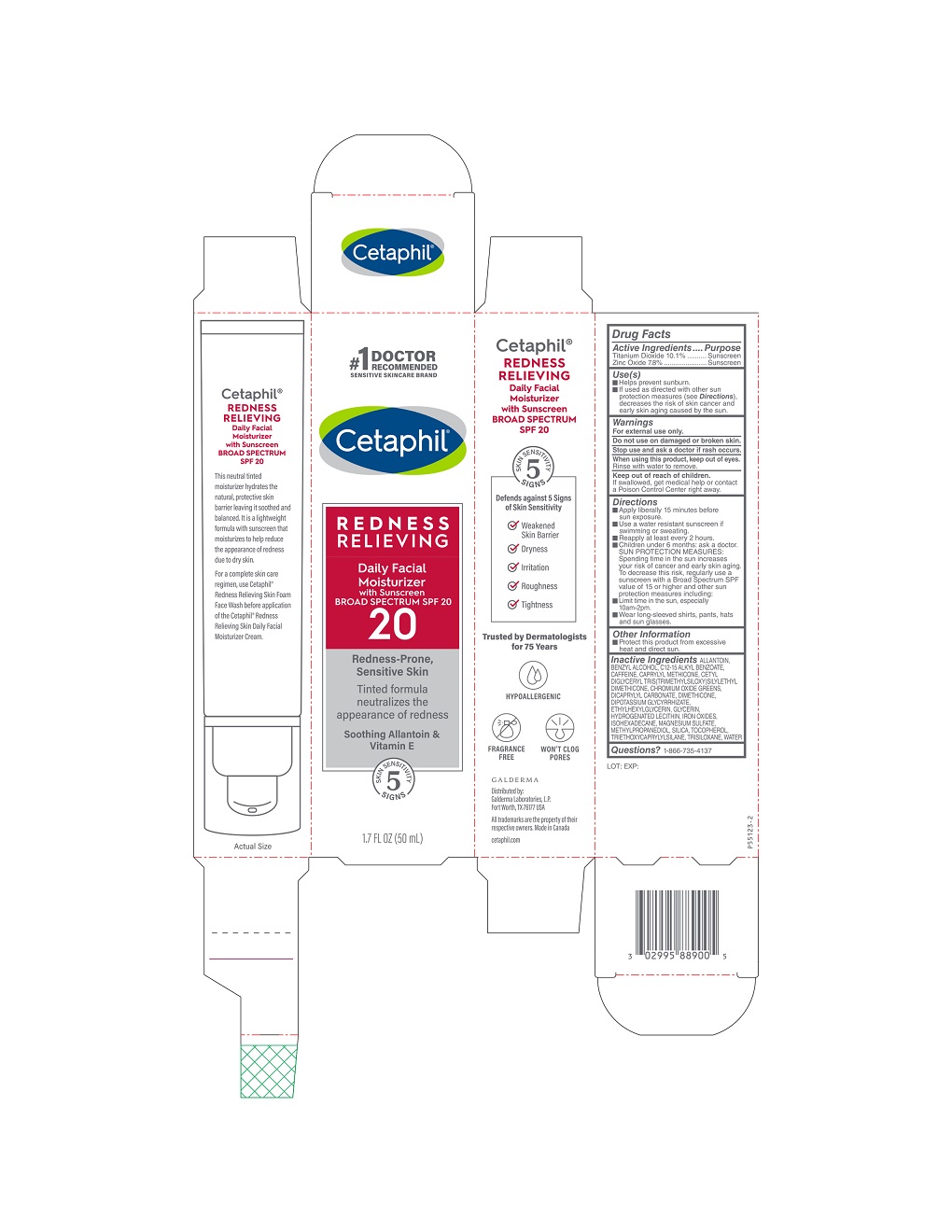
#1 Doctor Recommended
Sensitive Skincare Brand
Cetaphil®
REDNESS
RELIEVING
Daily Facial
Moisturizer
with Sunscreen
BROAD SPECTRUM SPF 20
20
Redness-Prone,
Sensitive Skin
Tinted formula
neutralizes the
appearance of redness
Soothing Allantoin &
Vitamin E
5 Skin Sensitivity Signs
1.7 FL OZ (50 ML)
Distributed by:
Galderma Laboratories, L.P.
Fort Worth, TX 76177 USA
All trademarks are the property of their
respective owners.
Made in Canada
cetaphil.com
P55123-2