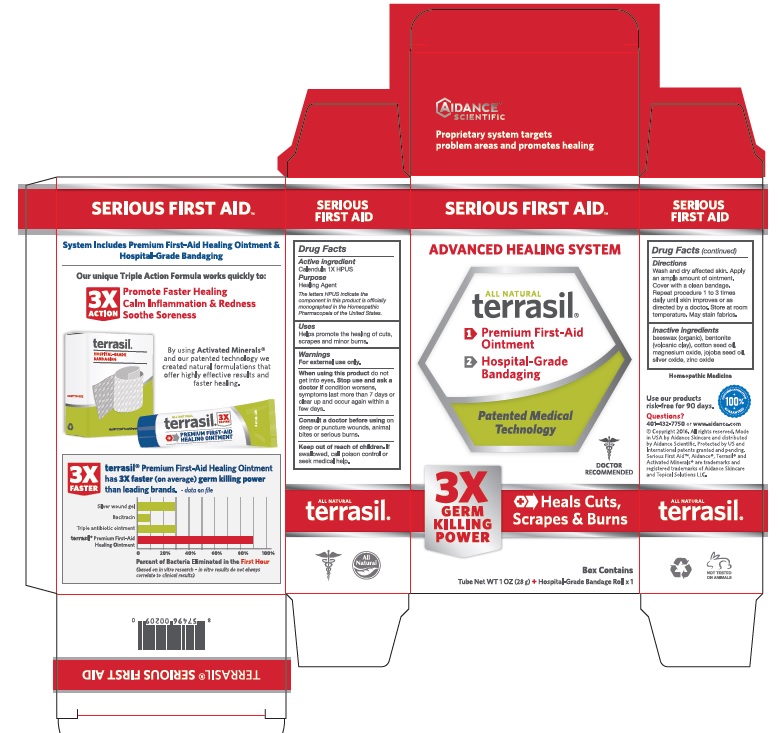
TERRASIL SERIOUS FIRST AID- calendula ointment
Aidance Skincare & Topical Solutions, LLC
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Active Ingredients
Calendula 1X HPUS
The letters HPUS indicate the component(s) in this product is (are) officially monographed in the Homeopathic Pharmacopeia of the United States.
Uses
Helps promote healing of cuts, scrapes and minor burns.
Warnings
For external use only. When using this product, do not get into eyes. Stop use and ask a doctor if condition worsens, symptoms last more than 7 days or clear up and occur again within a few days. Consult a doctor before using on deep or puncture wounds, animal bites or serious burns.
Keep out of reach of children.
If swallowed, call poison control or seek medical help.
Directions
Wash and dry affected skin. Apply an ample amount of ointment. Cover with a clean bandage. Repeat procedure 1 to 3 times daily until skin improves or as directed by a doctor. Store at room temperature. May stain fabrics.
Inactive Ingredients
beeswax (organic), bentonite (volcanic clay), cotton seed oil, jojoba seed oil, magnesium oxide, silver oxide, zinc oxide.
PRINCIPAL DISPLAY PANEL
SERIOUS FIRST AID
ADVANCED HEALING SYSTEM
All Natural
terrasil®
1> Premium First-Aid Ointment
2> Hospital-Grade Bandaging
Patented Medical Technology