DESCRIPTION
Ciclopirox gel 0.77% contains a synthetic antifungal agent, ciclopirox USP. It is intended for topical dermatologic use only.
Each gram of ciclopirox gel contains 7.70 mg of ciclopirox USP in a gel consisting of carbomer homopolymer, isopropyl alcohol, and medium chain triglycerides, sodium hydroxide, sodium lauryl sulfate, and purified water.
Ciclopirox gel is a white, slightly fluid gel.
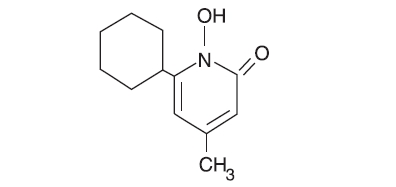
The chemical name for ciclopirox USP is 6-cyclohexyl-1-hydroxy-4-methyl-2(1H)-pyridinone, with the empirical formula C12H17NO2 and a molecular weight of 207.27. The CAS Registry Number is [29342-05-0]. The chemical structure is:
CLINICAL PHARMACOLOGY
Mechanism of Action
Ciclopirox is a hydroxypyridone antifungal agent although the relevance of this property for the indication of seborrheic dermatitis is not known. Ciclopirox acts by chelation of polyvalent cations (Fe3+ or Al3+), resulting in the inhibition of the metal-dependent enzymes that are responsible for the degradation of peroxides within the fungal cell.
Pharmacokinetics
A comparative study of the pharmacokinetics of ciclopirox gel and ciclopirox cream (ciclopirox olamine) 0.77% in 18 healthy males indicated that systemic absorption of ciclopirox from ciclopirox gel was higher than that of ciclopirox cream. A 5 gm dose of ciclopirox gel produced a mean (±SD) peak serum concentration of 25.02 (±20.6) ng/mL total ciclopirox and 5 gm of ciclopirox cream produced 18.62 (±13.56) ng/mL total ciclopirox. Approximately 3% of the applied ciclopirox was excreted in the urine within 48 hours after application, with a renal elimination half-life of about 5.5 hours.
In a study of ciclopirox gel, 16 men with moderate to severe tinea cruris applied approximately 15 grams/day of the gel for 14.5 days. The mean (±SD) dose- normalized values of Cmax for total ciclopirox in serum were 100 (±42) ng/mL on Day 1 and 238 (±144) ng/mL on Day 15. During the 10 hours after dosing on Day 1, approximately 10% of the administered dose was excreted in the urine.
Microbiology
Ciclopirox is a hydroxypyridinone antifungal agent that inhibits the growth of pathogenic dermatophytes. Ciclopirox has been shown to be active against most strains of the following microorganisms both in vitro and in clinical infections as described in the INDICATIONS AND USAGE section:
Trichophyton rubrum, Trichophyton mentagrophytes, and Epidermophyton floccosum
INDICATIONS AND USAGE
Superficial Dermatophyte Infections
Ciclopirox gel is indicated for the topical treatment of interdigital tinea pedis and tinea corporis due to Trichophyton rubrum, Trichophyton mentagrophytes, or Epidermophyton floccosum.
Seborrheic Dermatitis
Ciclopirox gel is indicated for the topical treatment of seborrheic dermatitis of the scalp.
CONTRAINDICATIONS
Ciclopirox gel is contraindicated in individuals who have shown hypersensitivity to any of its components.
WARNINGS
Ciclopirox gel is not for ophthalmic, oral, or intravaginal use.
Keep out of reach of children.
PRECAUTIONS
If a reaction suggesting sensitivity or chemical irritation should occur with the use of ciclopirox gel, treatment should be discontinued and appropriate therapy instituted. A transient burning sensation may occur, especially after application to sensitive areas. Avoid contact with eyes. Efficacy of ciclopirox gel in immunosuppressed individuals has not been studied. Seborrheic dermatitis in association with acne, atopic dermatitis, Parkinsonism, psoriasis and rosacea has not been studied with ciclopirox gel. Efficacy in the treatment of plantar and vesicular types of tinea pedis has not been established.
Information for Patients
The patient should be told the following:
- 1.
- Use ciclopirox gel as directed by the physician. Avoid contact with the eyes and mucous membranes. Ciclopirox gel is for external use only.
- 2.
- Use the medication for fungal infections for the full treatment time even though symptoms may have improved, and notify the physician if there is no improvement after 4 weeks.
- 3.
- A transient burning/stinging sensation may be felt. This may occur in approximately 15% to 20% of cases, when ciclopirox gel is used to treat seborrheic dermatitis of the scalp.
- 4.
- Inform the physician if the area of application shows signs of increased irritation or possible sensitization (redness with itching, burning, blistering, swelling, and/or oozing).
- 5.
- Avoid the use of occlusive dressings.
- 6.
- Do not use this medication for any disorder other than that for which it is prescribed.
Carcinogenesis, Mutagenesis, Impairment of Fertility
A 104-week dermal carcinogenicity study in mice was conducted with ciclopirox cream formulation applied at doses up to 1.93% (100 mg/kg/day or 300 mg/m2/day). No increase in drug related neopalsms was noted when compared to control.
The following in vitro genotoxicity tests have been conducted with ciclopirox: evaluation of gene mutation in the Ames Salmonella and E. coli assays (negative); chromosome aberration assays in V79 Chinese hamster lung fibroblast cells, with and without metabolic activation (positive); chromosome aberration assays in V79 Chinese hamster lung fibroblast cells in the presence of supplemental Fe3+, with and without metabolic activation (negative); gene mutation assays in the HGPRT-test with V79 Chinese hamster lung fibroblast cells (negative); and a primary DNA damage assay (i.e., unscheduled DNA synthesis assay in A549 human cells) (negative). An in vitro cell transformation assay in BALB/c 3T3 cells was negative for cell transformation. In an in vivo Chinese hamster bone marrow cytogenetic assay, ciclopirox was negative for chromosome aberrations at a dosage of 5000 mg/kg body weight.
A combined oral fertility and embryofetal developmental study was conducted in rats with ciclopirox olamine. No effect on fertility or reproductive performance was noted at the highest dose tested of 3.85 mg/kg/day ciclopirox (approximately 1.2 times the maximum recommended human dose based on body surface area comparisons).
Pregnancy
Teratogenic effects: Pregnancy Category B
There are no adequate or well-controlled studies in pregnant women. Therefore, ciclopirox gel should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Oral embryofetal developmental studies were conducted in mice, rats, rabbits and monkeys. Ciclopirox or ciclopirox olamine was orally administered during the period of organogenesis. No maternal toxicity, embryotoxicity or teratogenicity were noted at the highest doses of 77, 125, 80 and 38.5 mg/kg/day ciclopirox in mice, rats, rabbits and monkeys, respectively (approximately 11, 37, 51 and 24 times the maximum recommended human dose based on body surface area comparisons, respectively).
Dermal embryofetal developmental studies were conducted in rats and rabbits with ciclopirox olamine dissolved in PEG 400. Ciclopirox olamine was topically administered during the period of organogenesis. No maternal toxicity, embryotoxicity or teratogenicity were noted at the highest doses of 92 mg/kg/day and 77 mg/kg/day ciclopirox in rats and rabbits, respectively (approximately 27 and 49 times the maximum recommended human dose based on body surface area comparisons, respectively).
ADVERSE REACTIONS
In clinical trials, 140 (39%) of 359 subjects treated with ciclopirox gel reported adverse experiences, irrespective of relationship to test materials, which resulted in 8 subjects discontinuing treatment. The most frequent experience reported was skin burning sensation upon application, which occurred in approximately 34% of seborrheic dermatitis patients and 7% of tinea pedis patients. Adverse experiences occurring between 1% to 5% were contact dermatitis and pruritus. Other reactions that occurred in less than 1% included dry skin, acne, rash, alopecia, pain upon application, eye pain, and facial edema.
DOSAGE AND ADMINISTRATION
Superficial Dermatophyte Infections
Gently massage ciclopirox gel into the affected areas and surrounding skin twice daily, in the morning and evening immediately after cleaning or washing the areas to be treated. Interdigital tinea pedis and tinea corporis should be treated for 4 weeks. If a patient shows no clinical improvement after 4 weeks of treatment, the diagnosis should be reviewed.
Seborrheic Dermatitis of the Scalp
Apply ciclopirox gel to affected scalp areas twice daily, in the morning and evening for 4 weeks. Clinical improvement usually occurs within the first week with continuing resolution of signs and symptoms through the fourth week of treatment. If a patient shows no clinical improvement after 4 weeks of treatment, the diagnosis should be reviewed.
HOW SUPPLIED
Ciclopirox gel 0.77% is supplied in aluminium tubes and LDPE tubes.
30 g tubes (NDC 68462-455-35), 45 g tubes (NDC 68462-455-47) and 100 g tubes (NDC 68462-455-94).
Store at 20 to 25°C (68 to 77°F) [See USP Controlled Room Temperature].
Manufactured by:
Glenmark Pharmaceuticals Limited
Colvale-Bardez, Goa 403 513, India
Manufactured for:
Glenmark Pharmaceuticals Inc., USA
Mahwah, NJ 07430
Questions? 1 (888)721-7115
www.glenmarkpharma.com/usa
January 2017