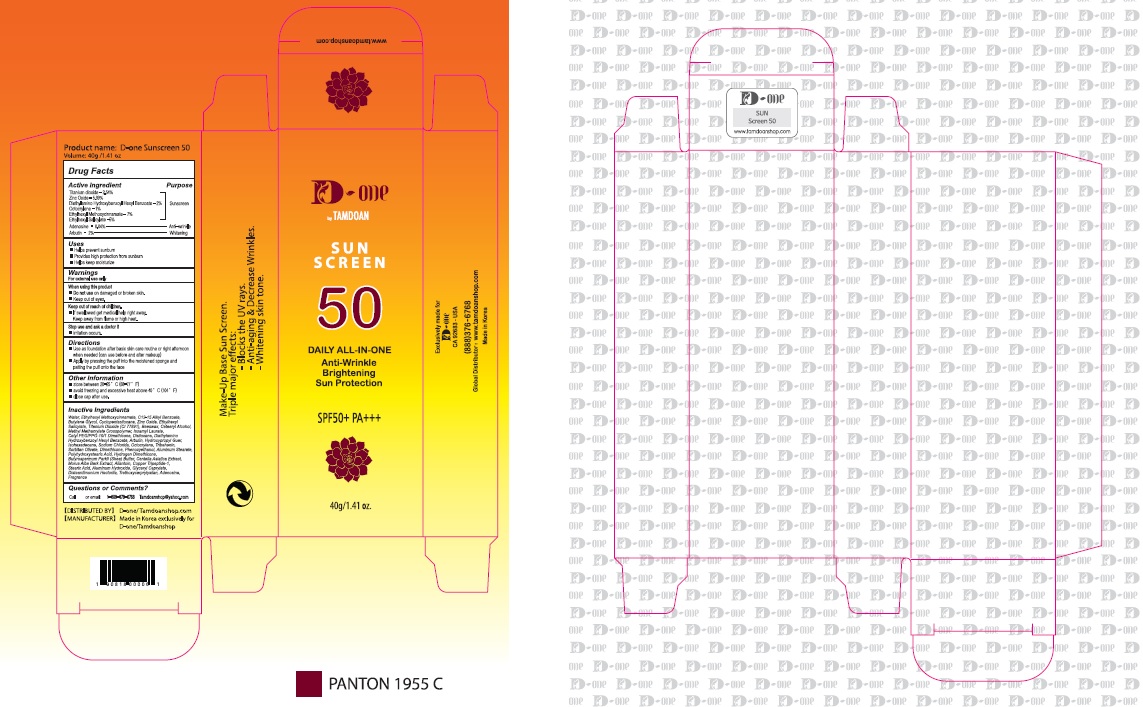
Titanium dioxide, Zinc Oxide, Diethylamino Hydroxybenzoyl Hexyl Benzoate, Octocrylene, Ethylhexyl Methoxycinnamate, Ethylhexyl Salicylate, Adenosine, Arbutin
- Use as foundation after basic skin care routine or right afternoon when needed (can use before and after makeup)
- Apply by pressing the puff into the moistened sponge and patting the puff onto the face
1. Do not use in the following cases(Eczema and scalp wounds)
2.Side Effects
1)Due to the use of this product if rash, irritation, itching and symptopms of hypersnesitivity occur dicontinue use and consult your pharmacist or doctor
3.General Precautions
1)If in contact with the eyes, wash out thoroughty with water If the symptoms are servere, seek medical advice immediately
2)This product is for exeternal use only. Do not use for internal use
4.Storage and handling precautions
1)If possible, avoid direct sunlight and store in cool and area of low humidity
2)In order to maintain the quality of the product and avoid misuse
3)Avoid placing the product near fire and store out in reach of children