Drug Facts
| Active Ingredient:
PEROX-A-MINT: | Purpose |
| Hydrogen Peroxide 1.5% | Oral Debriding Agent |
USES
Suction Swab with Perox-A-Mint Solution
- Aids in the removal of secretions and debris.
Suction Toothbrush CHG compatible*
- Aids in the removal of dental plaque, debris and secretions.
Suction Swab with Alcohol-Free Mouthwash
- Aids in the removal of debris.
- Helps the mouth feel fresh and clean.
- Helps temporarily reduce dryness.
Oropharyngeal Suction Catheter Non-sterile
- Aids in the removal of secretions from the oropharyngeal cavity only.
WARNINGS
Stop use and ask a doctor if:
- Sore mouth symptoms do not improve in 7 days.
- Swelling, rash or fever develops.
- Irritation, pain or redness persists or worsens.
Keep out of
reach of children.
If more than used for debriding
is accidentally swallowed, get medical help or contact a Poison Control
Center right away.
DIRECTIONS
Suction Swab with Perox-A-Mint Solution
- Before opening, turn package over, burst solution packet with thumbs.
- Peel lid to open.
- Remove Mouth Moisturizer and Applicator Swab.
- Attach Suction Swab to suction line.
- Clean teeth and oral cavity for approximately one minute.
- To suction, place thumb over port.
- To clear tubing, rinse with sterile saline or appropriate solution.
- Discard Suction Swab. Reattach Covered Yankauer to suction line.
- Place Mouth Moisturizer on Applicator Swab.
- Apply as needed to lips and inside mouth.
- Use up to 4 times daily or as directed by a dentist or doctor.
- Children under 12 years of age: supervise use.
- Children under 3 years of age: consult a dentist or doctor.
- Use a bite block when performing oral care on patients with altered levels of consciousness or those who cannot comprehend commands.
- Ensure foam is intact after use. If not, remove any particles from oral cavity.
Suction Toothbrush CHG compatible*
- Peel lid to open.
- Remove Suction Toothbrush and attach to suction line.
- When using with a cleansing solution, refer to the product packaging for indications, instructions and warnings.
- To suction, place thumb over port.
- To clear tubing, rinse with sterile saline or appropriate solution.
- Discard Suction Toothbrush. Reattach Covered Yankauer to suction line.
- Use Swab for additional cleansing as needed.
- Use two times daily or as directed by a dentist or doctor.
- Children under 12 years of age: supervise use.
- Children under 3 years of age: consult a dentist or doctor.
- Use a bite block when performing oral care on patients with altered levels of consciousness or those who cannot comprehend commands.
- Ensure foam is intact after use. If not, remove any particles from oral cavity.
Suction Swab with Alcohol-Free Mouthwash
- Before opening, turn package over, burst solution packet with thumbs.
- Peel lid to open.
- Remove Mouth Moisturizer and Applicator Swab.
- Attach Suction Swab to suction line.
- Clean teeth and oral cavity.
- To suction, place thumb over port.
- To clear tubing, rinse with sterile saline or appropriate solution.
- Discard Suction Swab. Reattach covered Yankauer to suction line.
- Place Mouth Moisturizer on Applicator Swab.
- Apply as needed to lips and inside mouth.
- Children under 12 years of age: supervise use.
- Use a bite block when performing oral care on patients with altered levels of consciousness or those who cannot comprehend commands.
- Ensure foam is intact after use. If not, remove any particles from oral cavity.
Oropharyngeal Suction Catheter Non-sterile
- Attach Suction Catheter to suction line.
- Suction secretions from the oropharyngeal cavity.
- To suction, place thumb over port.
- To clear tubing, rinse with sterile saline or appropriate solution.
- Discard Suction Catheter. Reattach Covered Yankauer to suction line.
- Use a bite block when performing oral care on patients with altered levels of consciousness or those who cannot comprehend commands.
Oropharyngeal Suction Catheter Non-sterile
Caution
- Federal (U.S.A.) law restricts this device to sale by or on the order of a physician or licensed practitioner.
Inactive Ingredients
Suction Swab with
Perox-A-Mint Solution
Water, menthol flavor, polysorbate
80, phosphoric acid, sodium saccharin, Blue 1 (CI 42090), Yellow 6
(CI 15985)
DESCRIPTION
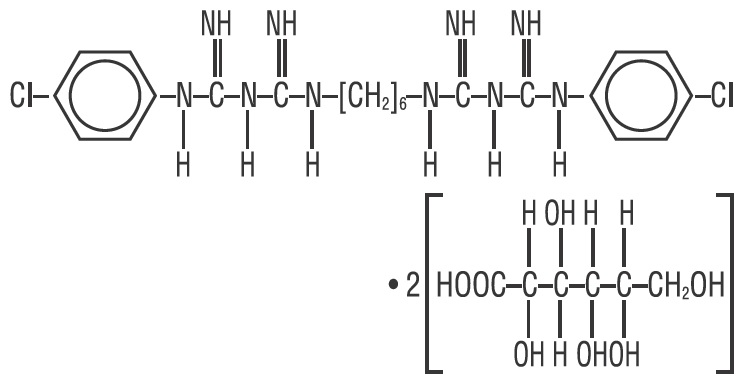
Chlorhexidine Gluconate is an oral rinse containing 0.12% chlorhexidine gluconate (1,11-hexamethylene bis[5-(p-chlorophenyl) biguanide] di-D-gluconate) in a base containing water, 11.6% alcohol, glycerin, PEG-40 sorbitan diisostearate, flavor, sodium saccharin, and FD&C Blue No. 1. Chlorhexidine Gluconate is a near-neutral solution (pH range 5-7). Chlorhexidine Gluconate is a salt of chlorhexidine and gluconic acid. Its chemical structure is:
CLINICAL PHARMACOLOGY
Chlorhexidine Gluconate Oral Rinse provides antimicrobial activity during oral rinsing. The clinical significance of Chlorhexidine Gluconate Oral Rinse’s antimicrobial activities is not clear. Microbiological sampling of plaque has shown a general reduction of counts of certain assayed bacteria, both aerobic and anaerobic, ranging from 54-97% through six months use.
Use of Chlorhexidine Gluconate Oral Rinse in a six month clinical study did not result in any significant changes in bacterial resistance, overgrowth of potentially opportunistic organisms or other adverse changes in the oral microbial ecosystem. Three months after Chlorhexidine Gluconate Oral Rinse was discontinued, the number of bacteria in plaque had returned to baseline levels and resistance of plaque bacteria to chlorhexidine gluconate was equal to that at baseline.
PHARMACOKINETICS
Pharmacokinetic studies with Chlorhexidine Gluconate Oral Rinse indicate approximately 30% of the active ingredient, chlorhexidine gluconate, is retained in the oral cavity following rinsing. This retained drug is slowly released in the oral fluids. Studies conducted on human subjects and animals demonstrate chlorhexidine gluconate is poorly absorbed from the gastrointestinal tract. The mean plasma level of chlorhexidine gluconate reached a peak of 0.206 µg/g in humans 30 minutes after they ingested a 300 mg dose of the drug. Detectable levels of chlorhexidine gluconate were not present in the plasma of these subjects 12 hours after the compound was administered. Excretion of chlorhexidine gluconate occurred primarily through the feces (~90%). Less than 1% of the chlorhexidine gluconate ingested by these subjects was excreted in the urine.
INDICATIONS AND USAGE
Chlorhexidine Gluconate Oral Rinse is indicated for use between dental visits as part of a professional program for the treatment of gingivitis as characterized by redness and swelling of the gingivae, including gingival bleeding upon probing. Chlorhexidine Gluconate Oral Rinse has not been tested among patients with acute necrotizing ulcerative gingivitis (ANUG). For patients having coexisting gingivitis and periodontitis, see PRECAUTIONS.
CONTRAINDICATIONS
Chlorhexidine Gluconate Oral Rinse should not be used by persons who are known to be hypersensitive to chlorhexidine gluconate or other formula ingredients.
WARNINGS
The effect of Chlorhexidine Gluconate Oral Rinse on periodontitis has not been determined. An increase in supragingival calculus was noted in clinical testing in Chlorhexidine Gluconate Oral Rinse users compared with control users. It is not known if Chlorhexidine Gluconate Oral Rinse use results in an increase in subgingival calculus. Calculus deposits should be removed by a dental prophylaxis at intervals not greater than six months. Anaphylaxis, as well as serious allergic reactions, have been reported during postmarketing use with dental products containing chlorhexidine, see CONTRAINDICATIONS.
PRECAUTIONS
GENERAL
- For patients having coexisting gingivitis and periodontitis, the presence or absence of gingival inflammation following treatment with Chlorhexidine Gluconate Oral Rinse should not be used as a major indicator of underlying periodontitis.
- Chlorhexidine Gluconate Oral Rinse can cause staining of oral surfaces, such as tooth surfaces, restorations, and the dorsum of the tongue. Not all patients will experience a visually significant increase in toothstaining. In clinical testing, 56% of Chlorhexidine Gluconate Oral Rinse users exhibited a measurable increase in facial anterior stain, compared to 35% of control users after six months; 15% of Chlorhexidine Gluconate Oral Rinse users developed what was judged to be heavy stain, compared to 1% of control users after six months. Stain will be more pronounced in patients who have heavier accumulations of unremoved plaque. Stain resulting from use of Chlorhexidine Gluconate Oral Rinse does not adversely affect health of the gingivae or other oral tissues. Stain can be removed from most tooth surfaces by conventional professional prophylactic techniques. Additional time may be required to complete the prophylaxis. Discretion should be used when prescribing to patients with anterior facial restorations with rough surfaces or margins. If natural stain cannot be removed from these surfaces by a dental prophylaxis, patients should be excluded from Chlorhexidine Gluconate Oral Rinse treatment if permanent discoloration is unacceptable. Stain in these areas may be difficult to remove by dental prophylaxis and on rare occasions may necessitate replacement of these restorations.
- Some patients may experience an alteration in taste perception while undergoing treatment with Chlorhexidine Gluconate Oral Rinse. Rare instances of permanent taste alteration following Chlorhexidine Gluconate Oral Rinse use have been reported via post-marketing product surveillance.
PREGNANCY: TERATOGENIC EFFECTS
Pregnancy Category B. Reproduction Studies have been performed in rats and rabbits at chlorhexidine gluconate doses up to 300 mg/kg/day and 40 mg/kg/day respectively, and have not revealed evidence of harm to fetus. However, adequate and well-controlled studies in pregnant women have not been done. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
NURSING MOTHERS
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Chlorhexidine Gluconate Oral Rinse is administered to nursing women. In parturition and lactation studies with rats, no evidence of impaired parturition or of toxic effects to suckling pups was observed when chlorhexidine gluconate was administered to dams at doses that were over 100 times greater than that which would result from a person’s ingesting 30 mL of Chlorhexidine Gluconate Oral Rinse per day.
PEDIATRIC USE
Clinical effectiveness and safety of Chlorhexidine Gluconate Oral Rinse have not been established in children under the age of 18.
CARCINOGENESIS, MUTAGENESIS, IMPAIRMENT OF FERTILITY
In a drinking water study in rats, carcinogenic effects were not observed at doses up to 38 mg/kg/day. Mutagenic effects were not observed in two mammalian in vivo mutagenesis studies with chlorhexidine gluconate. The highest doses of chlorhexidine used in a mouse dominant-lethal assay and a hamster cytogenetics test were 1000 mg/kg/day and 250 mg/kg/day, respectively. No evidence of impaired fertility was observed in rats at doses up to 100 mg/kg/day.
ADVERSE REACTIONS
The most common side effects associated with chlorhexidine gluconate oral rinses are: 1) an increase in staining of teeth and other oral surfaces; 2) an increase in calculus formation; and 3) an alteration in taste perception; see WARNINGS and PRECAUTIONS. Oral irritation and local allergy-type symptoms have been spontaneously reported as side effects associated with use of chlorhexidine gluconate rinse. The following oral mucosal side effects were reported during placebo-controlled adult clinical trials: aphthous ulcer, grossly obvious gingivitis, trauma, ulceration, erythema, desquamation, coated tongue, keratinization, geographic tongue, mucocele, and short frenum. Each occurred at a frequency of less than 1.0%. Among post marketing reports, the most frequently reported oral mucosal symptoms associated with Chlorhexidine Gluconate Oral Rinse are stomatitis, gingivitis, glossitis, ulcer, dry mouth, hypesthesia, glossal edema, and paresthesia. Minor irritation and superficial desquamation of the oral mucosa have been noted in patients using Chlorhexidine Gluconate Oral Rinse. There have been cases of parotid gland swelling and inflammation of the salivary glands (sialadenitis) reported in patients using Chlorhexidine Gluconate Oral Rinse.
OVERDOSAGE
Ingestion of 1 or 2 ounces of Chlorhexidine Gluconate Oral Rinse by a small child (~10 kg body weight) might result in gastric distress, including nausea, or signs of alcohol intoxication. Medical attention should be sought if more than 4 ounces of Chlorhexidine Gluconate Oral Rinse is ingested by a small child or if signs of alcohol intoxication develop.
DOSAGE AND ADMINISTRATION
Chlorhexidine Gluconate Oral Rinse therapy should be initiated directly following a dental prophylaxis. Patients using Chlorhexidine Gluconate Oral Rinse should be reevaluated and given a thorough prophylaxis at intervals no longer than six months. Recommended use is twice daily oral rinsing for 30 seconds, morning and evening after toothbrushing. Usual dosage is 15 mL of undiluted Chlorhexidine Gluconate Oral Rinse. Patients should be instructed to not rinse with water, or other mouthwashes, brush teeth, or eat immediately after using Chlorhexidine Gluconate Oral Rinse. Chlorhexidine Gluconate Oral Rinse is not intended for ingestion and should be expectorated after rinsing.
HOW SUPPLIED
Chlorhexidine Gluconate Oral Rinse is supplied as a blue liquid in single dose 0.5 fluid ounce (15mL) amber plastic bottles with child-resistant dispensing closures. STORE at 20°C to 25°C (68°F to 77°F), excursions permitted to 15°C to 30°C (59°F to 86°F) [See USP controlled room temperature].