Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- •
- runny nose
- •
- itchy, watery eyes
- •
- sneezing
- •
- itching of the nose or throat
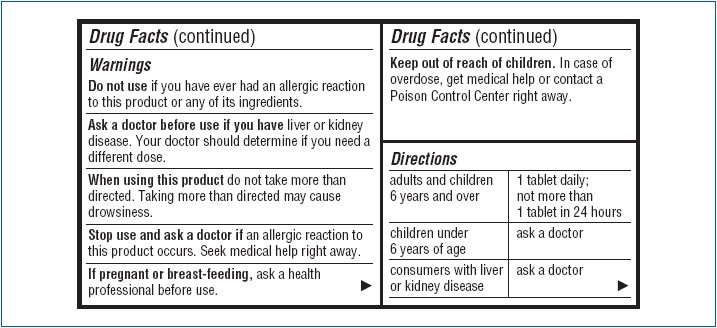
Ask a doctor before use if you have
liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
do not take more than directed. Taking more than directed may cause drowsiness.
Stop use and ask a doctor if
an allergic reaction to this product occurs. Seek medical help right away.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
|
adults and children 6 years and over |
1 tablet daily; not more than 1 tablet in 24 hours |
|
children under 6 years of age |
ask a doctor |
|
consumers with liver of kidney disease |
ask a doctor |
Other information
- •
- Tamper Evident: do not use if foil seal under cap is missing, open or broken.
- •
- store between 20° to 25°C (68° to 77°F)
- •
- protect from excessive moisture
Questions or comments?
call 1-877-446-3679 (1-877-4-INFO-RX)
Manufactured for:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
Made in India
Code No.: MH/DRUGS/25/NKD/89
PRODUCT PACKAGING
NDC 0378-8880-10
Original Prescription Strength
Non-Drowsy*
Loratadine
Tablets USP, 10 mg
Antihistamine
Indoor and Outdoor Allergies
24 Hour Relief of:
- •
- Sneezing
- •
- Runny Nose
- •
- Itchy, Watery Eyes
- •
- Itchy Throat or Nose
*When taken as directed. See Drug Facts Panel.
RMX8880C1
100 Tablets