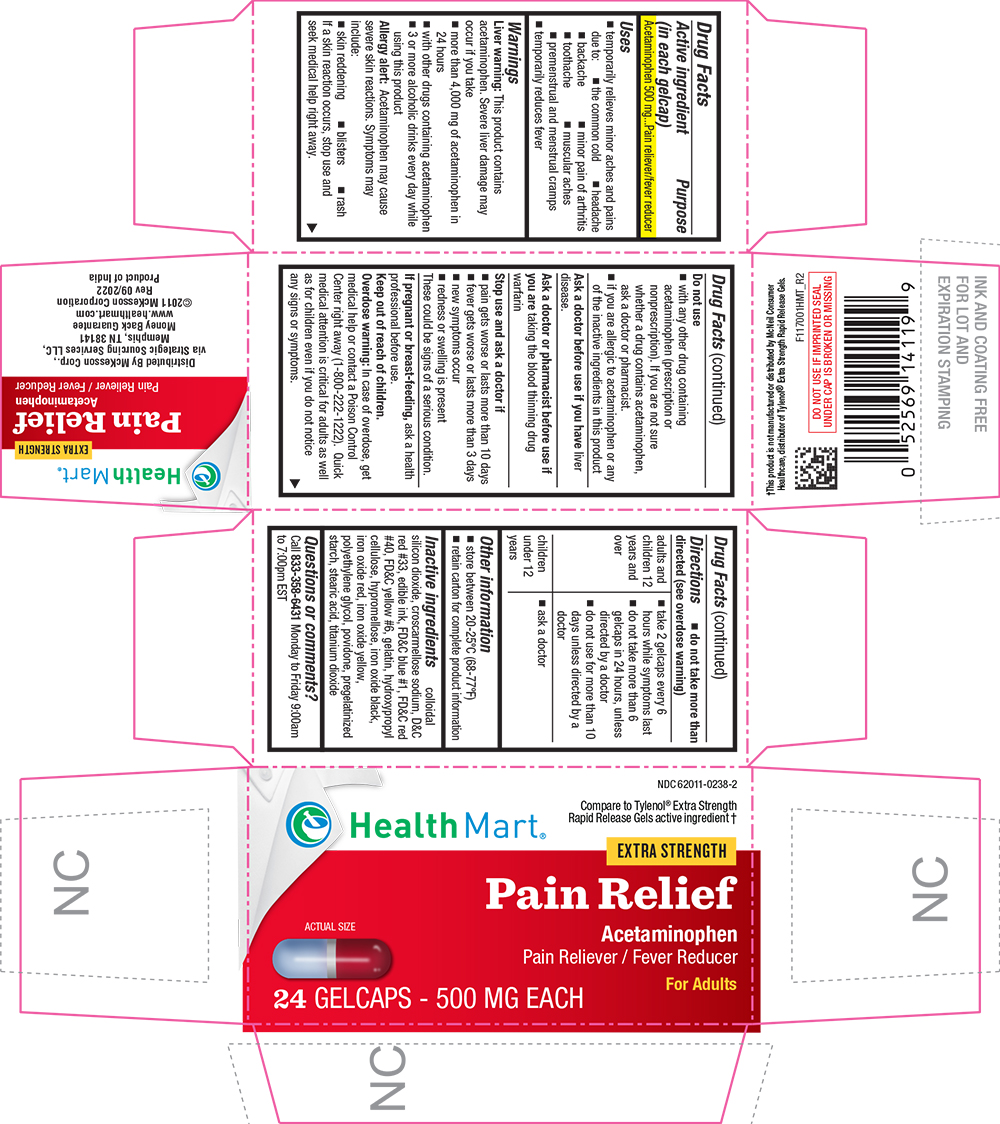
Uses
- temporarily relieves minor aches and pains due to:
- the common cold
- headache
- backache
- minor pain of arthritis
- toothache
- muscular aches
- premenstrual and menstrual cramps
- temporarily reduces fever
Warnings
Liver warning
This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert
Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are allergic to acetaminophen or any of the inactive ingredients in this product
Directions
- do not take more than directed (see overdose warning)
| adults and children 12 years and over |
|
| children under 12 years |
|
Inactive ingredients
colloidal silicon dioxide, croscarmellose sodium, D&C red #33, edible ink, FD&C blue #1, FD&C red #40, FD&C yellow #6, gelatin, hydroxypropyl cellulose, hypromellose, iron oxide black, iron oxide red, iron oxide yellow, polyethylene glycol, povidone, pregelatinized starch, stearic acid, titanium dioxide