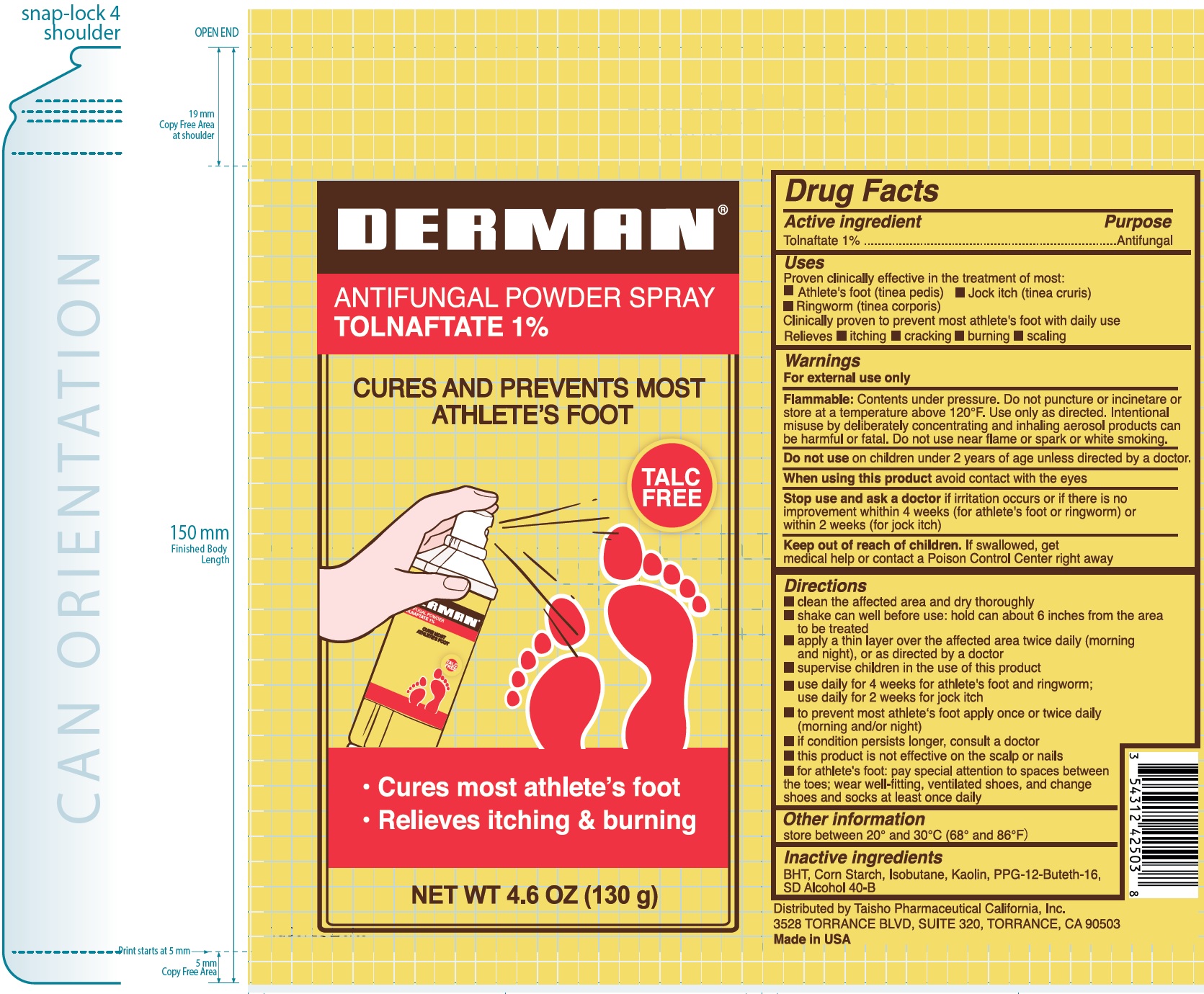
Uses
Proven clinically effective in the treatment of most:
Clinically proven to prevent most athlete's foot with daily use Relieves
- Athlete's foot (tinea pedis)
- Jock itch (tine cruris)
- Ringworm (tinea corporis)
- itching
- cracking
- burning
- scaling
Warnings
For external use only
Contents under pressure. Do not puncture or incinetare or store at a temperature above 120°F. Use only as directed. Intentional misuse by deliberately concentrating and inhaling aerosol products can be harmful or fatal. Do not use near flame or spark or white smoking. Flammable:
Directions
- clean the affected area and dry thoroughly
- shake can well before use: hold can about 6 inches from the area to be treated
- apply a thin layer over the affected area twice daily (morning and night), or as directed by a doctor
- supervise children in the use of this product
- use daily for 4 weeks for athlete's foot and ringworm; use daily for 2 weeks for jock itch
- to prevent most athlete's foot apply once or twice daily (morning and/or night)
- if condition persists longer, consult a doctor
- this product is not effective on the scalp or nails
- for athlete's foot: pay special attention to spaces between the toes; wear well-fitting, ventilated shoes, and change shoes and socks at least once daily