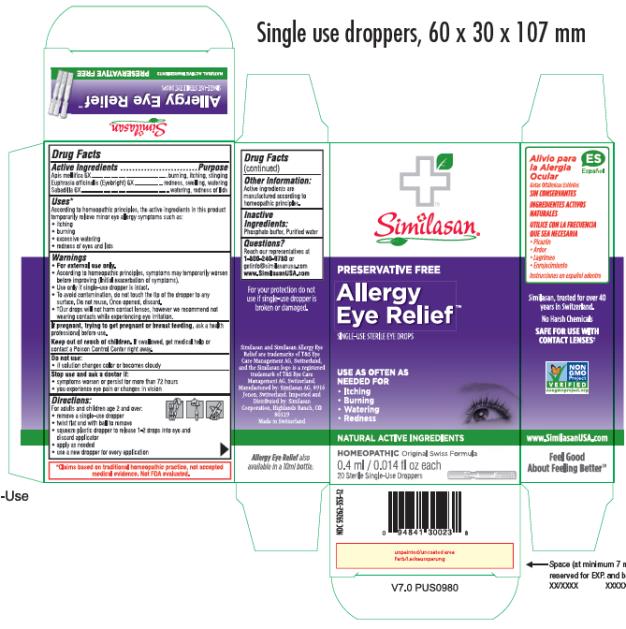
ALLERGY EYE RELIEF- apis mellifera and euphrasia stricta and schoenocaulon officinale seed solution/ drops
Similasan Corporation
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Active Ingredients
Apis mellifica 6X
Euphrasia officinalis (Eyebright) 6X
Sabadilla 6X
Purpose
burning, itching, stinging
redness, swelling, watering
watering, redness of lids
Uses*
According to homeopathic principles, the active ingredients in this product temporarily relieve minor symptoms such as:
• itching
• burning
• excessive watering
Warnings
• For external use only.
• According to homeopathic principles, symptoms may temporarily worsen before improving (Initial exacerbation of symptoms).
• Use only if single-use dropper is intact.
• To avoid contamination, do not touch the tip of the dropper to any surface. Do not reuse. Once opened, discard.
• †Our drops will not harm contact lenses, however we recommend not wearing contacts while experiencing eye irritation.
If pregnant, trying to get pregnant or breast feeding,
ask a health professional before use.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Do not use:
• if solution changes color or becomes cloudy
Stop use and ask a doctor if:
• symptoms worsen or persist for more than 72 hours
• you experience eye pain or changes in vision
Directions
For adults and children age 2 and over:
• remove a single-use dropper
• twist flat end with ball to remove
• squeeze plastic dropper to release 1-2 drops into eye and discard applicator
• apply as needed
• use a new dropper for every application
Other Information
Active ingredients are manufactured according to homeopathic principles.
Inactive Ingredients
Phosphate buffer, Purified water
Questions?
Reach our representatives at 1-800-240-9780 or getinfo@similasanusa.com
www.SimilasanUSA.com
PRINCIPAL DISPLAY PANEL
Allergy Eye
Relief
SINGLE-USE STERILE EYE DROPS
0.4 ml/0.014 fl oz each