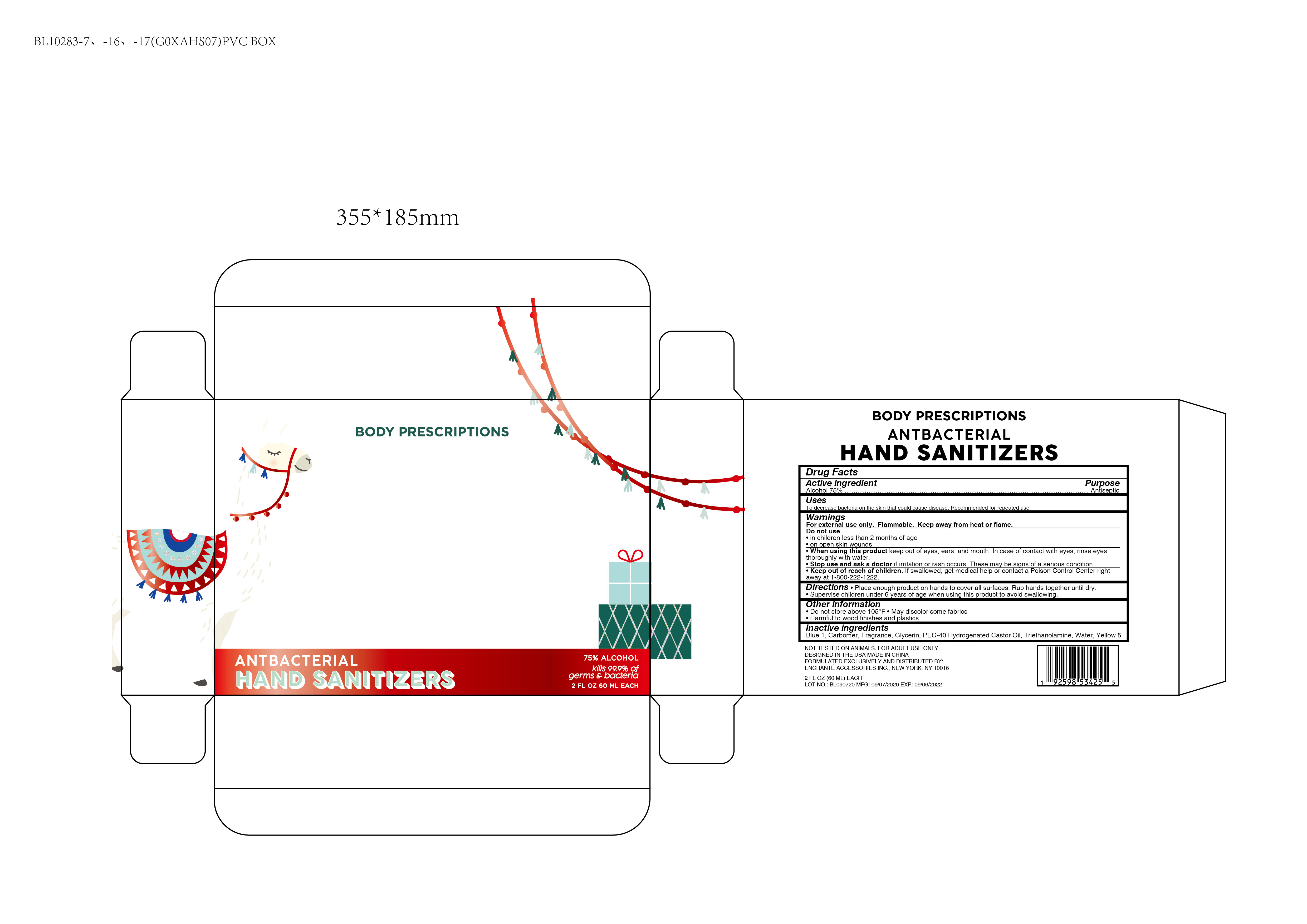
BODY PRESCRIPTIONS Cranberry HAND SANITIZER ANTIBACTERIAL
BODY PRESCRIPTIONS Cranberry HAND SANITIZER ANTIBACTERIAL
WARNINGS
For external use only
Flammable. Keep away from heat or flame
When using this product, keep out of eyes, ears, and mouth
In case of contact with eyes, rinse eyes thoroughly with water
Do not use
- in children less than two months of age
- on open skin wounds
Directions
Place enough product on hands to cover all surfaces. Rub hands together until dry.
Supervise children under 6 years of age when using this product to avoid swallowing
Other information
Do not store above 105F
May discolor some fabrics
Harmful to wood finishes and plastics
Inactive ingredients
Blue 1, Glycerin, PEG-40 Hydrogenated Castor Oil, Carbomer, Triethanolamine, Water, Yellow 5
BODY PRESCRIPTIONS Winter Berry HAND SANITIZER ANTIBACTERIAL
BODY PRESCRIPTIONS Winter Berry HAND SANITIZER ANTIBACTERIAL
Warnings
For external use only
Flammable. Keep away from heat or flame
When using this product, keep out of eyes, ears, and mouth
In case of contact with eyes, rinse eyes thoroughly with water
Do not use
- in children less than two months of age
- on open skin wounds
Directions
Place enough product on hands to cover all surfaces. Rub hands together until dry.
Supervise children under 6 years of age when using this product to avoid swallowing
Other Information
Do not store above 105F
May discolor some fabrics
Harmful to wood finishes and plastics
Inactive Ingredients
Blue 1, Water, Glycerin, PEG-40 Hydrogenated Castor Oil, Carbomer, Triethanolamine, Yellow 5
BODY PRESCRIPTIONS Peppermint HAND SANITIZER ANTIBACTERIAL
BODY PRESCRIPTIONS Peppermint HAND SANITIZER ANTIBACTERIAL
Warnings
For external use only
Flammable. Keep away from heat or flame
When using this product, keep out of eyes, ears, and mouth
In case of contact with eyes, rinse eyes thoroughly with water
Do not use
- in children less than two months of age
- on open skin wounds
Directions
Place enough product on hands to cover all surfaces. Rub hands together until dry.
Supervise children under 6 years of age when using this product to avoid swallowing
Other Information
Do not store above 105F
May discolor some fabrics
Harmful to wood finishes and plastics