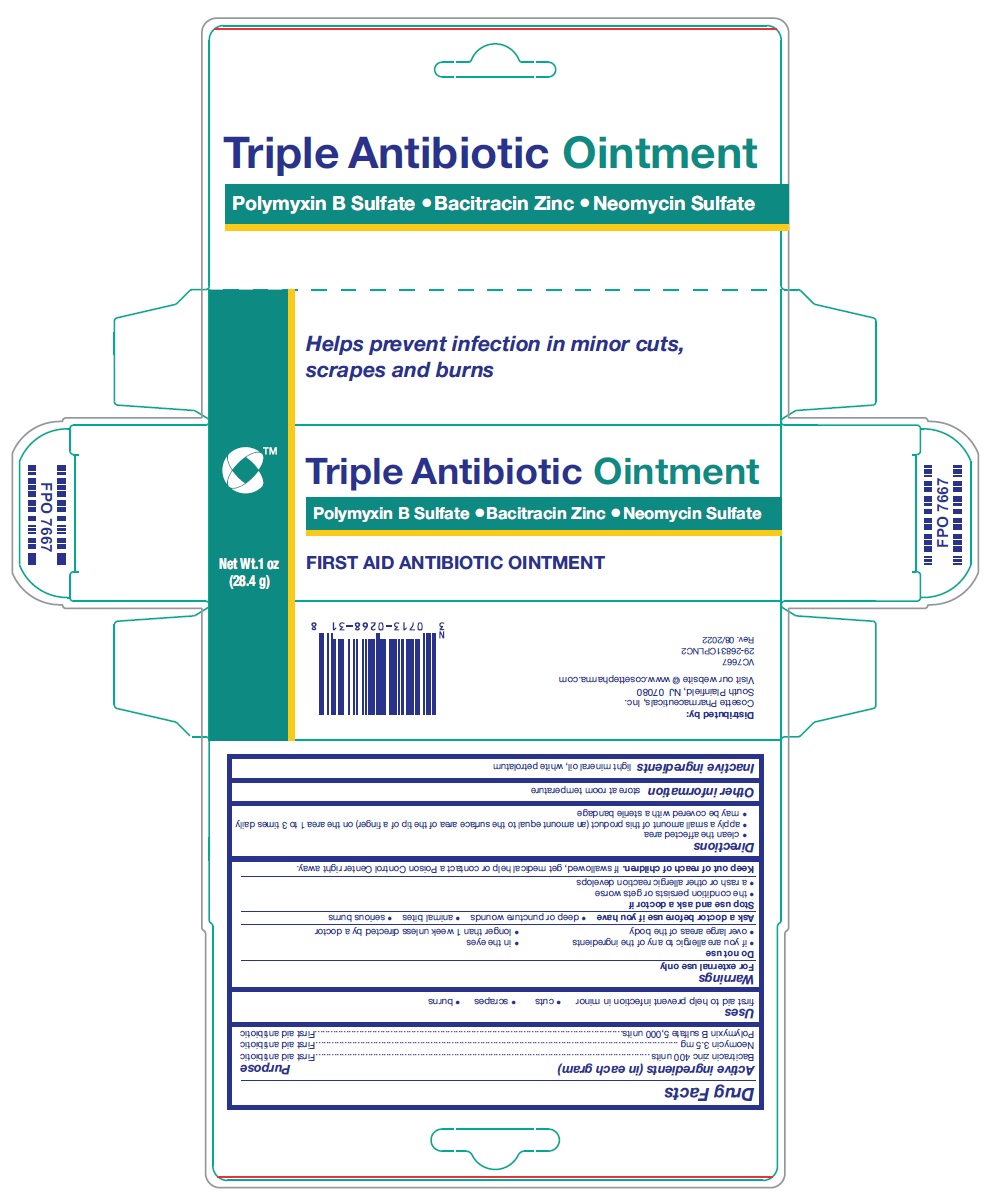
ACTIVE INGREDIENT (in each gram)
Bacitracin zinc 400 units
Neomycin 3.5 mg
Polymyxin B sulfate 5,000 units
WARNINGS
For external use only
Do not use
• if you are allergic to any of the ingredients
• in the eyes
• over large areas of the body
• longer than 1 week unless directed by a doctor
Ask a doctor before use if you have
• deep or puncture wounds
• animal bites
• serious burns
Stop use and ask a doctor if
• the condition persists or gets worse
• a rash or other allergic reaction develops
KEEP OUT OF REACH OF CHILDREN
If swallowed, get medical help or contact a Poison Control Center right away.