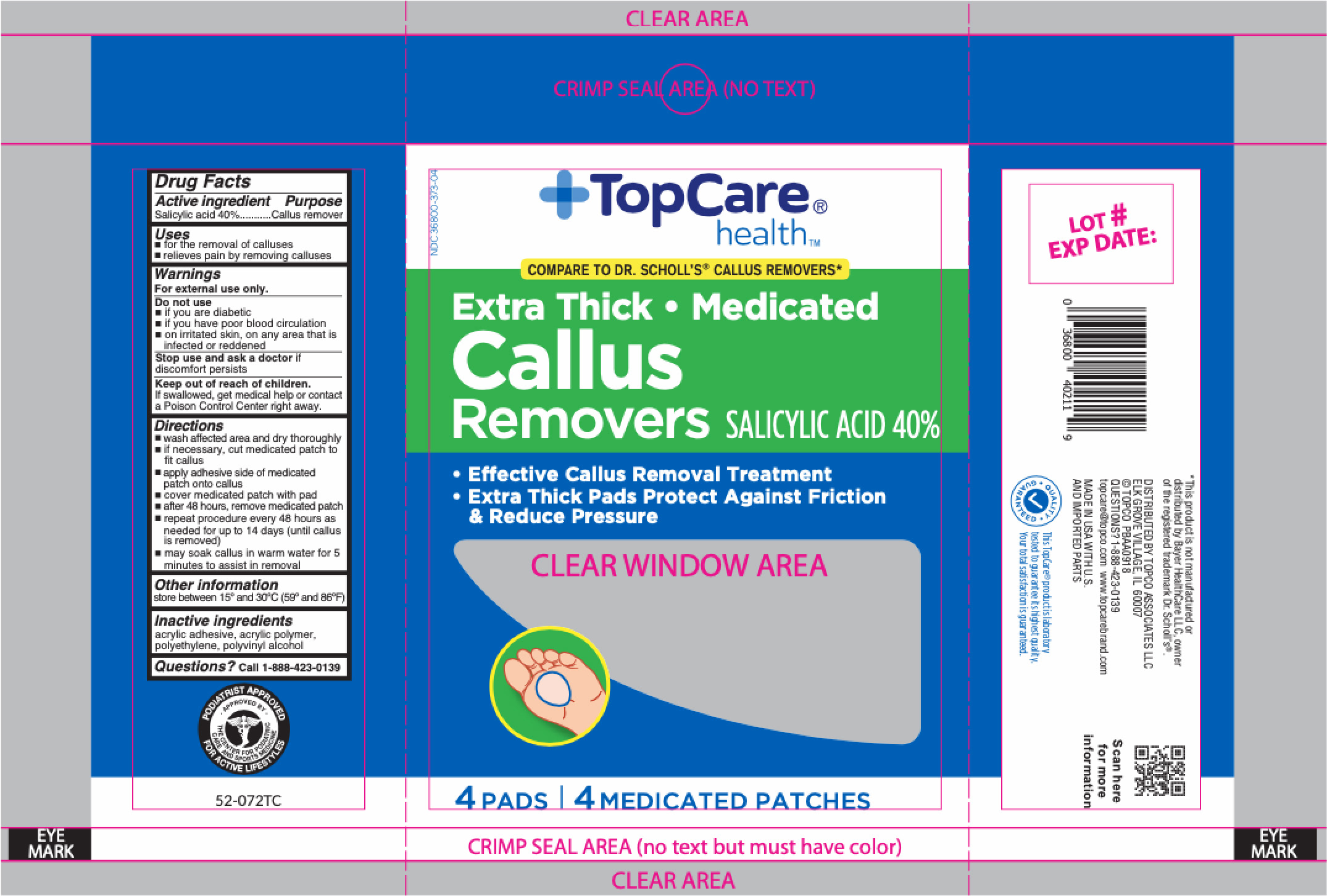
Active ingredient
Salicylic acid 40%
Use
- for the removal of calluses
- relieves pain by removing calluses
Warnings
For external use only.
Do not use
- if you are a diabetic
- if you have poor blood circulation
- on irritated skin, on any area that is infected or reddened
Stop use and ask a doctor
if discomfort persists
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- wash affected area and dry area thoroughly
- if necessary, cut medicated patch to fit callus
- apply adhesive side down of medicated patch onto callus
- cover medicated patch with pad
- after 48 hours, remove medicated patch
- repeat procedure every 48 hours as needed for up to 14 days (until corn is removed)
- may soak corn in warm water for 5 minutes to assist in removal
Other information
store between 15°C to 30°C (59°F to 86°F)
Inactive ingredients
acrylic adhesive, acrylic polymer, polyethylene, polyvinyl alcohol
Questions?
call 1-866-964-0939
Principal Display Panel
TopCare
health
Extra Thick • Medicated
Callus
Removers SALICYLIC ACID 40%
- Effective Callus Removal Treatment
- Extra Thick Pads Protect Against Friction
& Reduce Pressure
4 PADS | 4 MEDICATED PATCHES