FULL PRESCRIBING INFORMATION
WARNING: LOSS OF BONE MINERAL DENSITY
- Women who use depo-subQ provera 104 may lose significant bone mineral density. Bone loss is greater with increasing duration of use and may not be completely reversible [see Warnings and Precautions (5.1)].
- It is unknown if use of depo-subQ provera 104 during adolescence or early adulthood, a critical period of bone accretion, will reduce peak bone mass and increase the risk for osteoporotic fracture in later life [see Warnings and Precautions (5.1)].
- Depo-subQ provera 104 is not recommended as a long-term (i.e., longer than 2 years) birth control method or medical therapy for endometriosis-associated pain unless other options are considered inadequate [see Indications and Usage (1) and Warnings and Precautions (5.1)].
1 INDICATIONS AND USAGE
Depo-subQ provera 104 is indicated in females of reproductive age for:
- Prevention of pregnancy and
- Management of endometriosis-associated pain.
Limitations of Use:
The use of depo-subQ provera 104 is not recommended as a long-term (i.e., longer than 2 years) birth control method or medical therapy for endometriosis-associated pain unless other options are considered inadequate [see Dosage and Administration (2.1) and Warnings and Precautions (5.1)].
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions
Depo-subQ provera 104 is only for subcutaneous administration and is only to be administered by a healthcare professional.
Use for longer than 2 years is not recommended (unless other birth control methods or medical therapies for endometriosis-associated pain are considered inadequate) due to the impact of long-term depo-subQ provera 104 treatment on bone mineral density (BMD) [see Warnings and Precautions (5.1)].
Prior to the first injection confirm that the patient is not pregnant. For women who are sexually active and who have regular menses, administer the first injection only during the first 5 days of a normal menstrual period. For women who are breast-feeding, administer the first injection during or after the sixth postpartum week.
The recommended dosage of depo-subQ provera 104 is 104 mg given subcutaneously every 12 to 14 weeks. If more than 14 weeks elapse between injections, confirm that the patient is not pregnant before the next injection. Instruct the patient that if they are unable to receive an injection within 12–14 weeks, another contraceptive method should be used until the next depo-subQ provera 104 injection. The dosage does not need to be adjusted for body weight.
Inject the entire contents of the pre-filled syringe using strict aseptic technique into the upper anterior thigh or abdomen, rotating the sites with every injection [see Dosage and Administration (2.3)].
2.2 Switching from Another Method of Contraception
When switching from another contraceptive method to depo-subQ provera 104, administer depo-subQ provera 104 in a manner that ensures continuous contraceptive coverage. Follow the respective recommendations when switching from the contraceptive methods listed below:
- Combined hormonal contraceptives: administer the first injection of depo-subQ provera 104 within 7 days after the last day of using the combined hormonal contraceptive method (i.e., within 7 days after taking the last active pill).
- An implant: administer the first injection of depo-subQ provera 104 on the day of implant removal.
- A contraceptive vaginal ring or transdermal system: administer the first injection of depo-subQ provera 104 on the day the patient would have inserted the next ring or applied the next transdermal system.
- An Intrauterine Device (IUD) or Intrauterine System (IUS): administer the first injection of depo-subQ provera 104 on the day of IUD/IUS removal. If the IUD/IUS is not removed on the first day of the patient's menstrual cycle, instruct patients to use a non-hormonal back-up method of birth control for the first 7 days after administration of depo-subQ provera 104.
- Depot medroxyprogesterone acetate injectable suspension for intramuscular use (DMPA-IM): inject depo-subQ provera 104 12 to 14 weeks after the last dose of DMPA-IM.
2.3 Preparation and Administration Instructions
Prior to injection:
- Ensure all the components in Figure A are available and that depo-subQ provera 104 is at room temperature.
- Shake the pre-filled syringe vigorously prior to injection to ensure appropriate viscosity of the suspension.
- Inspect depo-subQ provera 104 visually for particulate matter and discoloration.
Figure A. Components in the Package
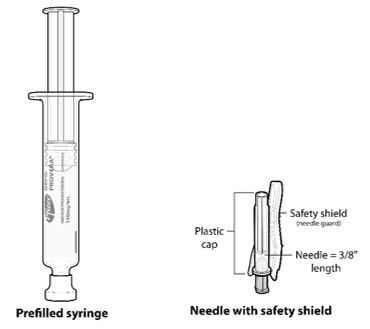
| Step 1: Select & Prepare the Injection Area | |
| Figure B. Preferred injection areas: 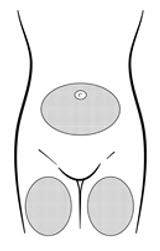 Left or right upper thigh or abdomen |
| Step 2: Prepare Syringe | |
| Figure C.
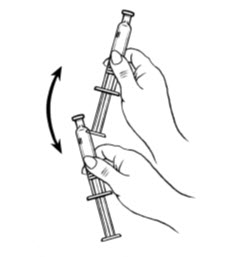 Shake vigorously for 1 minute |
| Figure D.
 |
| Figure E.
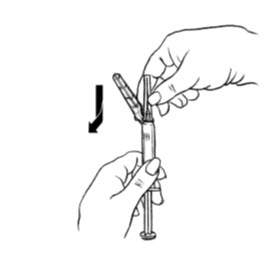 |
| Figure F.
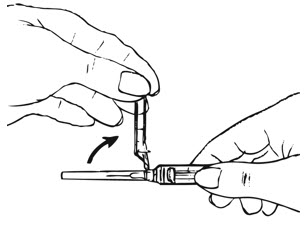 |
| Figure G.
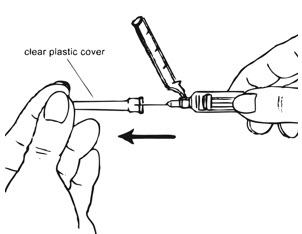 |
| Figure H.
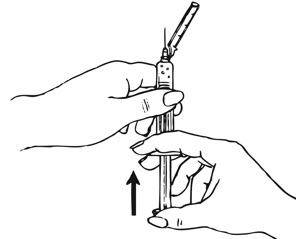 |
| Step 3: Injecting depo-Sub Q provera 104 | |
| Figure I.
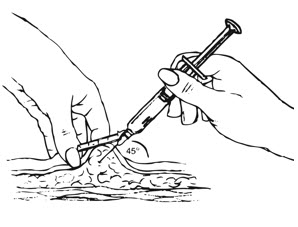 |
Inject slowly until the syringe is empty (Figure J).
| Figure J. Inject slowly (5–7 seconds) 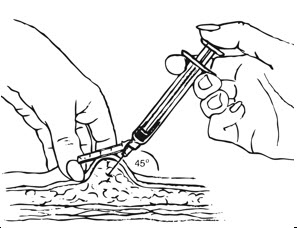 |
| Step 4: Remove the Needle and Activate the Safety Shield | |
| Figure K.
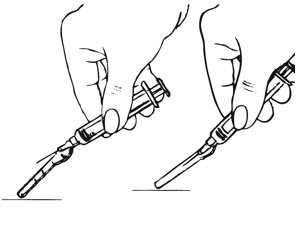 |
| Figure L.
 |
3 DOSAGE FORMS AND STRENGTHS
Injectable suspension (104 mg/0.65 mL) in a single-dose pre-filled syringe, packaged with a 26-gauge × 3/8-inch Terumo SurGuard® needle.
4 CONTRAINDICATIONS
The use of depo-subQ provera 104 is contraindicated in the following conditions:
- Active thrombophlebitis, or current or history of thromboembolic disorders, or cerebral vascular disease [see Warnings and Precautions (5.2)].
- Known, suspected, or past malignancy of the breast [see Warnings and Precautions (5.3)].
- Significant liver disease [see Warnings and Precautions (5.13)].
- Known hypersensitivity to medroxyprogesterone acetate or any of the ingredients in depo-subQ provera 104 [see Warnings and Precautions (5.5)].
- Undiagnosed vaginal bleeding [see Warnings and Precautions (5.11)].
5 WARNINGS AND PRECAUTIONS
5.1 Loss of Bone Mineral Density
Use of depo-subQ provera 104 reduces serum estrogen levels and is associated with significant loss of bone mineral density (BMD). This loss of BMD is of particular concern during adolescence and early adulthood, a critical period of bone accretion. It is unknown if use of depo-subQ provera 104 by younger women will reduce peak bone mass and increase the risk for osteoporotic fracture in later life.
A study to assess the reversibility of loss of BMD in adolescents was conducted with DMPA-IM. After discontinuing DMPA-IM in these adolescents, mean BMD loss at the total hip and femoral neck did not fully recover by 5 years (60 months) post-treatment in the sub-group of adolescents who were treated for more than 2 years [see Clinical Studies (14.4)]. Similarly, in adults, there was only partial recovery of mean BMD at the total hip, femoral neck, and lumbar spine towards baseline by 2 years post-treatment [see Clinical Studies (14.3)].
The use of depo-subQ provera 104 is not recommended as a long-term (i.e., longer than 2 years) birth control method or medical therapy for endometriosis-associated pain unless other options are considered inadequate. BMD should be evaluated when a woman needs to continue to use depo-subQ provera 104 long-term. In adolescents, interpretation of BMD results should take into account patient age and skeletal maturity.
Other birth control methods or therapies for endometriosis-associated pain should be considered in the risk/benefit analysis for the use of depo-subQ provera 104 in women with osteoporosis risk factors. Depo-subQ provera 104 can pose an additional risk in patients with risk factors for osteoporosis (e.g., metabolic bone disease, chronic alcohol and/or tobacco use, anorexia nervosa, strong family history of osteoporosis, or chronic use of drugs that can reduce bone mass such as anticonvulsants or corticosteroids).
5.2 Arterial and Venous Thromboembolic Disorders
There have been reports of serious arterial and venous thrombotic events in women treated with DMPA-IM. Women with a history of thromboembolic disorders were not studied in clinical trials of depo-subQ provera 104. Although no causal relationship between the use of depo-subQ provera 104 and thrombotic events has been clearly established, patients who develop arterial or venous thrombosis while taking depo-subQ provera 104 should discontinue treatment.
Do not re-administer depo-subQ provera 104 pending examination if there is a sudden onset of a suspected vascular ocular event (e.g., partial or complete loss of vision, proptosis, or diplopia) or migraine. Do not re-administer depo-subQ provera 104 if examination reveals papilledema or retinal vascular lesions.
5.3 Cancer Risks
Breast Cancer
The use of hormonal contraceptives, including depo sub-Q provera 104, is contraindicated in women who have or have had breast cancer because breast cancer may be sensitive to hormones [see Contraindications (4)]. Women who have a family history of breast cancer or a significant risk of breast cancer should be monitored.
The results of five large case-control studies assessing the association between DMPA-IM use and the risk of breast cancer are summarized in Figure M. Three of the studies suggest a slightly increased risk of breast cancer in the overall population of users; these increased risks were statistically significant in one study. One US study1 evaluated the timing and duration of use and found a statistically significant increased risk of breast cancer in recent DMPA-IM users (defined as last use within the past five years) who used DMPA-IM for 12 months or longer; this is consistent with results of a previous study2.
| Figure M. Risk Estimates of Breast Cancer in DMPA-IM Users |
|---|
| Odds ratio estimates were adjusted for the following covariates: Lee et al. (1987): age, parity, and socioeconomic status. Paul et al. (1989): age, parity, ethnic group, and year of interview. WHO (1991): age, center, and age at first live birth. Shapiro et al. (2000): age, ethnic group, socioeconomic status, and any combined estrogen/progestogen oral contraceptive use. Li et al. (2012): age, year, BMI, duration of OC use, number of full-term pregnancies, family history of breast cancer, and history of screening mammography. |
| Odds Ratio [95% confidence interval (CI)] displayed on logarithmic scale |
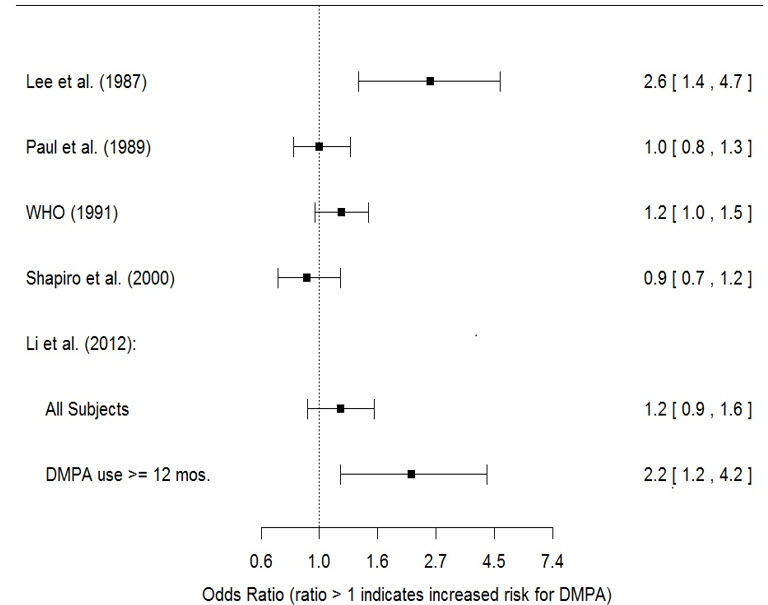 |
Based on the published SEER-18 2015 incidence rate (age-adjusted to the 2000 US Standard Population) of breast cancer for US women, all races, age 20 to 49 years, a doubling of risk would increase the incidence of breast cancer in women who use DMPA-IM from about 73 to about 146 cases per 100,000 women.
Other Cancers
The relative rate of invasive squamous-cell cervical cancer in women who ever used DMPA-IM was estimated to be 1.11 (95% CI: 0.96 to 1.29). No trends in risk with duration of use or times since initial or most recent exposure were observed.
Long-term, case-controlled surveillance of users of DMPA-IM found no overall increased risk of ovarian or liver cancer.
5.4 Ectopic Pregnancy
Healthcare professionals should be alert to the possibility of an ectopic pregnancy among women using depo-subQ provera 104 who become pregnant or complain of severe abdominal pain.
5.5 Anaphylaxis
Serious anaphylactic reactions have been reported in women using depo-subQ provera 104. If an anaphylactic reaction occurs, appropriate emergency medical treatment should be administered.
5.6 Fluid Retention
Because progestational drugs including depo-subQ provera 104 may cause fluid retention, monitor patients with conditions that might be affected by fluid retention.
5.7 Weight Gain
Weight gain is a common occurrence in women using depo-subQ provera 104. In three large clinical trials using depo-subQ provera 104, the mean weight gain was 3.5 lb (1.6 kg) in the first year of use. In a small, two-year study comparing depo-subQ provera 104 to DMPA-IM, the mean weight gain observed for women using depo-subQ provera 104 [7.5 lb (3.4 kg)] was similar to the mean weight gain for women using DMPA-IM [7.6 lb (3.5 kg)].
Although there are no data related to weight gain beyond 2 years for depo-subQ provera 104, the data on DMPA-IM may be relevant. In a clinical study, after five years, 41 women using Depo-Provera CI (150 mg) had a mean weight gain of 11.2 lb (5.1 kg), while 114 women using non-hormonal contraception had a mean weight gain of 6.4 lb (2.9 kg).
5.8 Delayed Return of Ovulation or Fertility
Return to ovulation is likely to be delayed after stopping depo-subQ provera 104, as demonstrated in a study of 15 women who received multiple doses of depo-subQ provera 104:
- Median time to ovulation was 10 months after the last injection.
- Earliest return to ovulation was 6 months after the last injection.
- 12 women (80%) ovulated within 1 year of the last injection.
However, ovulation has occurred as early as 14 weeks after a single dose of depo-subQ provera 104; therefore, administer the next depo-subQ provera 104 12 to 14 weeks after the last injection.
Return to fertility also is likely to be delayed after stopping therapy. Among 28 women using depo-subQ provera 104 for contraception who stopped treatment to become pregnant, 7 women were lost to follow-up. One woman became pregnant within one year of her last injection and another woman became pregnant 443 days after her last injection. The remaining 19 women had not become pregnant; it is not known if these 19 women were still attempting to become pregnant or if they had started a new contraceptive method.
5.9 Depression
Depression (3% of depo-subQ provera 104-treated patients) and other mood disorders have been reported in clinical trials of depo-subQ provera 104 [see Adverse Reactions (6.1)]. Patients with a history of depression or who are on treatment for depression may be at increased risk for depression recurrence or exacerbation and for associated mood disorders while receiving depo-subQ provera 104. Therefore, patients should be monitored for symptoms of depression and mood changes.
5.10 Injection Site Reactions
In five clinical studies of depo-subQ provera 104 involving 2325 women (282 treated for up to 6 months, 1780 treated for up to 1 year, and 263 women treated for up to 2 years), 5% of women reported injection site reactions, and 1% had persistent skin changes (small areas of induration or atrophy).
In post-marketing experience, injection site reactions such as persistent atrophy of the injection site, dimpling/indentation, and injection site lump/nodule have been reported.
5.11 Bleeding Irregularities
Most women using depo-subQ provera 104 experienced changes in menstrual bleeding patterns, such as amenorrhea, irregular unpredictable spotting or bleeding, prolonged spotting or bleeding, or heavy bleeding [see Adverse Reactions (6.1)]. Fewer women experienced irregular bleeding and more experienced amenorrhea with longer term use of depo-subQ provera 104, consistent with expected endometrial thinning effects.
In three contraception trials, 39% of 2053 depo-subQ provera 104-treated women experienced amenorrhea during Month 6, and 57% experienced amenorrhea during Month 12. In two endometriosis trials using depo-subQ provera 104, 24% of 289 women experienced amenorrhea during Month 6 [see Adverse Reactions (6.1)].
If abnormal bleeding is persistent or severe, evaluate the patient for underlying pathology or pregnancy.
5.12 Risk of Hyperglycemia in Patients with Diabetes
Some patients receiving progestins may exhibit a decrease in glucose tolerance; therefore, patients with diabetes may be at greater risk of hyperglycemia.
5.13 Jaundice and Elevated Transaminase
Discontinue depo-subQ provera 104 if jaundice or elevated transaminase levels develop. Depo-subQ provera 104 may be resumed after both the jaundice and elevated transaminase levels resolve, and the healthcare professional determines that depo-subQ provera 104 did not cause the abnormalities.
6 ADVERSE REACTIONS
The following important adverse reactions are described in more detail in other sections of the prescribing information:
- Loss of bone mineral density [see Warnings and Precautions (5.1)]
- Arterial and venous thromboembolic disorders [see Warnings and Precautions (5.2)]
- Anaphylaxis [see Warnings and Precautions (5.5)]
- Fluid retention [see Warnings and Precautions (5.6)]
- Delayed return of ovulation or fertility [see Warnings and Precautions (5.8)]
- Depression [see Warnings and Precautions (5.9)]
- Injection site reactions [see Warnings and Precautions (5.10)]
- Bleeding irregularities [see Warnings and Precautions (5.11)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure to depo-subQ provera 104 in five clinical trials involving 2325 women including 2043 women who received treatment for contraception (1780 treated up to 1 year and 263 treated for up to 2 years) and 282 women for endometriosis for up to 6 months. In these pooled trials, 9% of women discontinued treatment due to an adverse reaction and the most common reason for discontinuation was dysfunctional uterine bleeding (3%).
Adverse Reactions in the Contraception Adult Studies
Table 1 presents frequently reported adverse reactions (>1%) in the contraception pooled studies. In these studies, the most frequently reported adverse reactions (>5%) were dysfunctional uterine bleeding (e.g., irregular, increased, decreased, or spotting), headache, increased weight, amenorrhea, and injection site reactions (e.g., pain/tenderness, nodule/lump, persistent atrophy/indentation/dimpling or lipodystrophy).
The frequency reported is based on the all-causality incidence in the pooled results of the three contraception studies. Closely related "Adverse Reaction" terms were grouped but individual patients reporting two or more grouped events were only counted once.
| Adverse Reaction | Frequency |
|---|---|
| Dysfunctional uterine bleeding (irregular, increase, decrease, spotting) | 18% |
| Headache | 9% |
| Increased weight (see below) | 7% |
| Amenorrhea | 6% |
| Injection site reactions (such as pain/tenderness, nodule/lump, persistent atrophy/indentation/dimpling, lipodystrophy) | 6% |
| Vaginitis, including candidiasis and bacterial | 5% |
| Abdominal pain | 4% |
| Urinary tract infections | 4% |
| Acne | 4% |
| Depression | 3% |
| Decreased libido | 3% |
| Nausea | 3% |
| Back pain | 3% |
| Breast pain/tenderness | 2% |
| Fatigue | 2% |
| Anxiety | 1% |
| Irritability | 1% |
| Dizziness | 1% |
Dysfunctional Uterine Bleeding
The extent of bleeding and spotting in the three contraception trials is presented in Figure N; data from the endometriosis trials are presented in Figure O [see Warnings and Precautions (5.1)].
| N=Number of subjects with bleeding or spotting during indicated month. |
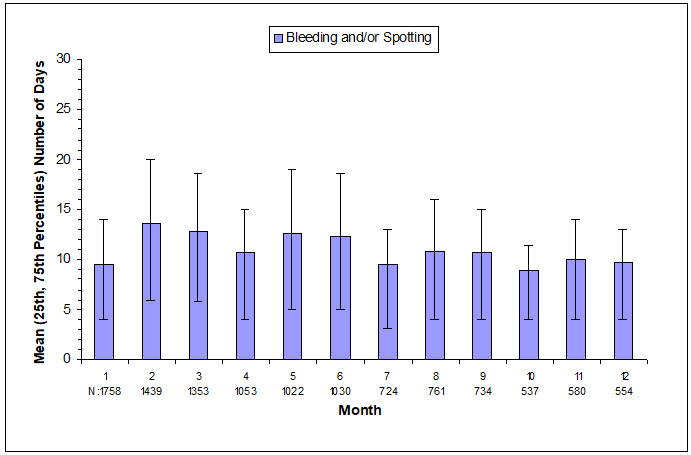 |
| N=Number of subjects with bleeding or spotting during indicated month. |
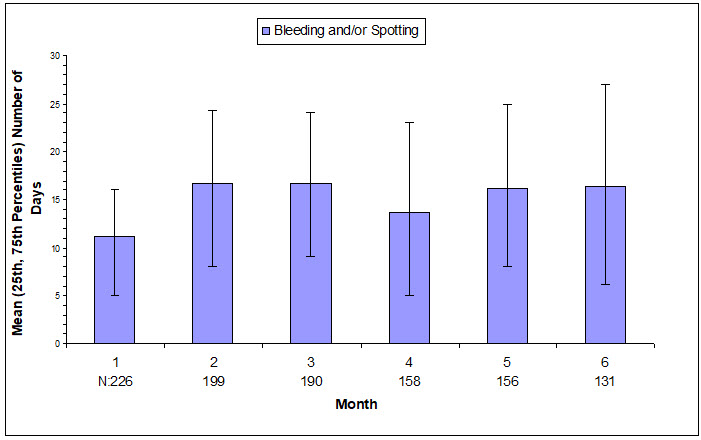 |
Weight Gain
In three large clinical trials, the mean weight gain in depo-subQ provera 104 treated patients was 3.5 lb (1.6 kg) in the first year of use. Half (50%) of women remained within 4.9 lb (2.2 kg) of their initial body weight; 12% of women lost more than 4.9 lb (2.2 kg), and 38% of women gained more than 5.1 lb (2.3 kg). In a small, 2-year study comparing depo-subQ provera 104 to DMPA-IM, the mean weight gain observed for women using depo-subQ provera 104 [7.5 lb (3.4 kg)] was similar to the mean weight gain for women using DMPA-IM [7.7 lb (3.5 kg)].
Other Adverse Reactions Observed in Contraception Clinical Trials with depo-subQ provera 104
Other adverse reactions occurring at an incidence of <1% in women who received depo-subQ provera 104 were as follows:
- Neoplasms benign, malignant and unspecified (including cysts and polyps): breast lump
- Blood and lymphatic system disorders: anemia
- Immune system disorders: drug hypersensitivity
- Metabolism and nutrition disorders: weight decreased, fluid retention
- Nervous system disorders: facial palsy, syncope, paresthesia, somnolence
- Cardiac disorders: tachycardia
- Vascular disorders: hot flushes
- Respiratory, thoracic and mediastinal disorders: asthma, dyspnea
- Gastrointestinal disorders: diarrhea, abdominal distension
- Skin and subcutaneous tissue disorders: urticaria, pruritus, dry skin
- Reproductive system and breast disorders: dysmenorrhea, galactorrhea, dyspareunia
- General disorders and administration site conditions: chest pain
Adverse Reactions in the Endometriosis Adult Studies
The safety profile of depo-subQ provera 104 in endometriosis clinical trials was similar to the safety profile of depo-subQ provera 104 in the contraception studies with the exception of the following adverse reactions which were more frequently reported in patients with endometriosis: abdominal pain, diarrhea, nausea, and back pain.
In endometriosis studies, subjects recorded daily the occurrence and severity of hot flushes. Of the depo-subQ provera 104 users, 29% reported experiencing moderate or severe hot flushes at baseline, 36% at Month 3, and 27% at Month 6. Of the leuprolide users, 33% reported experiencing moderate or severe hot flushes at baseline, 74% at Month 3, and 69% at Month 6.
Adverse Reactions in the Adolescent Contraception Study
Depo-sub-Q provera 104 and DMPA-IM clinical trials reported similar safety profiles in adult study populations (see Table 1 above). Accordingly, a similar safety profile is expected for adolescents receiving depo-subQ provera 104 as for adolescents receiving DMPA-IM.
The safety profile of DMPA-IM for prevention of pregnancy in adolescents was observed to be generally similar to the safety profile of adult women using DMPA-IM for prevention of pregnancy, with the exception of the following adverse reactions which were reported more frequently by adolescents: abdominal pain, diarrhea, back pain, weight increased, depression, headache, and dysmenorrhea.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of DMPA-IM. Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
- Immune system disorders: anaphylactic reaction, anaphylactoid reaction, angioedema
- Vascular disorders: pulmonary embolism, deep vein thrombosis, thrombophlebitis
- Musculoskeletal and connective tissue disorders: osteoporosis (including osteoporotic fractures)
- Reproductive system and breast disorders: prolonged anovulation, unexpected pregnancy, uterine hyperplasia
- Respiratory, thoracic and mediastinal disorders: hoarseness
- Skin and subcutaneous tissue disorders: increased body odor
- Gastrointestinal disorders: gastrointestinal disturbances
- General disorders and administration site conditions: axillary swelling, chills, thirst
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on Depo-subQ provera 104
Moderate or Strong CYP3A Inducers
Concomitant use with moderate or strong CYP3A inducers may decrease concentrations of medroxyprogesterone acetate which may reduce depo-subQ provera 104 efficacy. This effect is based upon the primary metabolism of medroxyprogesterone acetate by CYP3A and was not confirmed by a clinical study.
Avoid coadministration of depo-subQ provera 104 with moderate or strong CYP3A inducers. Some examples of moderate CYP3A inducers are bosentan, efavirenz, etravirine, and modafinil. Some examples of strong CYP3A inducers are rifampin, carbamazepine, phenytoin, phenobarbital, mitotane, and St. John's wort (the CYP3A4 induction effect of St. John's wort varies widely and is preparation dependent). These examples are a guide and do not represent a comprehensive list of all possible drugs that may fit these categories.
The use of CYP3A inducers may require using a back-up or alternate contraceptive method.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
There is no use for depo-subQ provera 104 in pregnancy and therefore depo-subQ provera 104 should be discontinued during pregnancy. There appears to be little or no increased risk of birth defects in women who have inadvertently been exposed to medroxyprogesterone acetate injections in early pregnancy. Neonates exposed to medroxyprogesterone acetate in-utero and followed to adolescence showed no evidence of any adverse effects on their health including their physical, intellectual, sexual, or social development.
8.3 Nursing Mothers
Although medroxyprogesterone acetate is detectable in the milk of mothers receiving DMPA-IM, milk composition, quality, and amount do not appear to be adversely affected. Effects on milk production and lactation initiation/duration remain unclear when administered before 6 weeks after delivery. Neonates and infants exposed to medroxyprogesterone acetate from breast milk have been studied for developmental and behavioral effects through puberty, and no adverse effects have been noted.
8.4 Pediatric Use
Depo-subQ provera 104 is indicated for the prevention of pregnancy and management of endometriosis-associated pain in females of reproductive age. Efficacy is expected to be the same for post-menarchal females under the age of 17 as for users 17 years and older.
Use of depo-subQ provera 104 is associated with significant loss of bone mineral density (BMD). This loss of BMD is of particular concern during adolescence, a critical period of bone accretion. It is unknown if use of depo-subQ provera 104 by female adolescents will reduce peak bone mass and increase the risk for osteoporotic fractures in later life. In a study of adolescent females (12–18 years of age) receiving DMPA-IM for contraception, mean BMD 2 years after starting DMPA-IM decreased 1.9% (spine), 4.3% (total hip), and 4.2% (femoral neck). In those adolescents who used DMPA-IM for more than 2 years, mean BMD at total hip and femoral neck did not return to baseline within 5 years.
Depo-subQ provera 104 is not indicated before menarche.
11 DESCRIPTION
Depo-subQ provera 104 contains medroxyprogesterone acetate (MPA), a derivative of progesterone, as its active ingredient. MPA is a white to off-white, odorless crystalline powder that is stable in air and that melts between 205°C and 209°C. It is freely soluble in chloroform, soluble in acetone and dioxane, sparingly soluble in alcohol and methanol, slightly soluble in ether, and insoluble in water.
The chemical name for MPA is 17-hydroxy-6α-methylpregn-4-ene-3,20-dione 17-acetate. The structural formula is as follows:
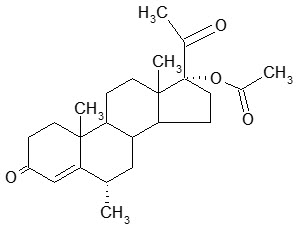
Depo-subQ provera 104 for subcutaneous use is available in pre-filled syringes, each containing 0.65 mL (104 mg) of sterile medroxyprogesterone acetate injectable suspension.
Each 0.65 mL contains the following inactive ingredients:
| Methylparaben | 1.040 mg |
| Propylparaben | 0.098 mg |
| Sodium Chloride | 5.200 mg |
| Polyethylene Glycol | 18.688 mg |
| Polysorbate 80 | 1.950 mg |
| Monobasic Sodium Phosphate H2O | 0.451 mg |
| Dibasic Sodium Phosphate 12H2O | 0.382 mg |
| Methionine | 0.975 mg |
| Povidone | 3.250 mg |
| Water for Injection | qs |
When necessary, the pH is adjusted with sodium hydroxide or hydrochloric acid, or both.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Depo-subQ provera 104 inhibits the secretion of gonadotropins, which primarily prevents follicular maturation and ovulation and causes thickening of cervical mucus. These actions contribute to its contraceptive effect.
Suppression of serum estradiol concentrations is likely to be responsible for the therapeutic effect on endometriosis-associated pain.
12.2 Pharmacodynamics
The following laboratory tests are expected to be affected by progestins including depo-subQ provera 104:
- Plasma and urinary steroid levels are decreased (e.g., progesterone, estradiol, pregnanediol, testosterone, cortisol).
- Gonadotropin levels are decreased.
- Sex-hormone-binding-globulin concentrations are decreased.
- Histology specimens may demonstrate changes consistent with progestin effects.
The following laboratory tests may be affected by depo-subQ provera 104, however the clinical significance is unknown:
- Protein-bound iodine and butanol extractable protein-bound iodine may increase.
- T3-uptake values may decrease.
- Coagulation test values for prothrombin (Factor II), and Factors VII, VIII, IX, and X may increase.
- Sulfobromophthalein and other liver function test values may be increased.
- The effects of medroxyprogesterone acetate on lipid metabolism are inconsistent. Both increases and decreases in total cholesterol, triglycerides, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol have been observed in studies.
12.3 Pharmacokinetics
The pharmacokinetic parameters of MPA following a single subcutaneous injection of depo-subQ provera 104 in healthy women (n=42) are shown in Table 2 and Figure P.
| Cmax
(ng/mL) | Tmax
(day) | C91
(ng/mL) | AUC0–91
(ng∙day/mL) | AUC0–∞
(ng∙day/mL) | t½ (day) |
|
|---|---|---|---|---|---|---|
| Abbreviations: Cmax=peak serum concentration; Tmax=time when Cmax is observed; C91=serum concentration at 91 days; AUC0–91 and AUC0–∞=area under the concentration-time curve over 91 days or infinity, respectively; t½=terminal half-life. | ||||||
| Mean | 1.56 | 8.8 | 0.402 | 66.98 | 92.84 | 43 |
| Min | 0.53 | 2.0 | 0.133 | 20.63 | 31.36 | 16 |
| Max | 3.08 | 80.0 | 0.733 | 139.79 | 162.29 | 114 |
Following subcutaneous administration of single depo-subQ provera 104 doses ranging from 50 to 150 mg (0.48 and 1.4 times the recommended dose, respectively), the AUC and Cmin (Day 91) increased with higher doses, but there was considerable overlap across dose levels. Serum MPA concentrations at Day 91 increased in a dose proportional manner but Cmax did not appear to increase proportionally with increasing dose. The AUC data were suggestive of dose linearity.
Absorption
Following a single subcutaneous injection of depo-subQ provera 104 in healthy women, serum MPA concentrations reached ≥0.2 ng/mL within 24 hours. The mean Tmax was attained approximately 1 week after injection.
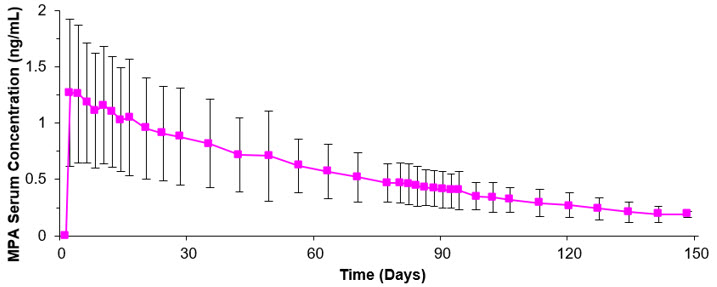 |
In a study to assess accumulation and the achievement of steady state following multiple subcutaneous administrations, trough concentrations of MPA were determined after 6, 12, and 24 months, and in a subset of 8 subjects, bi-weekly concentrations were determined within one dosing interval in the second year of administration. The mean (SD) MPA trough concentrations were 0.67 (0.36) ng/mL (n=157), 0.79 (0.36) ng/mL (n=144), and 0.87 (0.33) ng/mL (n=106) at 6, 12, and 24 months, respectively.
Depo-subQ provera 104 was administered subcutaneously into the anterior thigh or the abdomen to evaluate effects of injection site location on the MPA concentration-time profile. MPA trough concentrations (Cmin; Day 91) were similar for the two injection site locations.
Distribution
Plasma protein binding of MPA averages 86%. MPA binding occurs primarily to serum albumin. No binding of MPA occurs with sex-hormone-binding globulin (SHBG).
Elimination
Metabolism
MPA is extensively metabolized in the liver by P450 enzymes. Its metabolism primarily involves ring A and/or side-chain reduction, loss of the acetyl group, hydroxylation in the 2-, 6-, and 21-positions or a combination of these positions, resulting in more than 10 metabolites.
Excretion
Residual MPA concentrations at the end of the first dosing interval (12 to 14 weeks) of depo-subQ provera 104 were generally below 0.5 ng/mL, consistent with its apparent terminal half-life of ~40 days after subcutaneous administration. Most MPA metabolites were excreted in the urine as glucuronide conjugates with only small amounts excreted as sulfates.
Specific Populations
Racial Groups
There were no significant differences in the pharmacokinetics and/or pharmacodynamics of MPA after subcutaneous administration of depo-subQ provera 104 in African-American, Caucasian, and Asian women.
Effect of Body Weight
Although total MPA exposure was lower in obese women, no dosage adjustment of depo-subQ provera 104 is necessary based on body weight. The effect of body weight on the pharmacokinetics of MPA following a single dose was assessed in a subset of women [n=42, body mass index (BMI) ranged from 18.2 to 46.7 kg/m2]. The AUC0–91 values for MPA were 71.6, 67.9, and 46.3 ng∙day/mL in women with BMI categories of ≤28 kg/m2, >28–38 kg/m2, and >38 kg/m2, respectively. The mean MPA Cmax was 1.74 ng/mL in women with BMI ≤28 kg/m2, 1.53 ng/mL in women with BMI >28–38 kg/m2, and 1.02 ng/mL in women with BMI >38 kg/m2, respectively. The MPA trough (Cmin) concentrations had a tendency to be lower in women with BMI >38 kg/m2.
14 CLINICAL STUDIES
14.1 Contraception Studies
In three open label clinical studies, depo-SubQ provera 104 (104 mg given every three months subcutaneously), was administered to healthy, sexually-active, nonpregnant women 18 to 49 years of age who desired long-term contraception. In these three studies, no pregnancies were detected among 2042 women treated with depo-subQ provera 104 for up to 1 year. In women less than 36 years of age (at baseline), the Pearl Index pregnancy rate in cycles in which no other contraceptive methods were used, was 0 pregnancies per 100 women-years of use (upper 95% CI = 0.25).
14.2 Endometriosis Studies
The efficacy of depo-subQ provera 104 in the reduction of endometriosis-associated pain in women with the signs and symptoms of endometriosis was demonstrated in two active comparator-controlled studies in pre-menopausal women 18 to 49 years of age with laparoscopically diagnosed endometriosis and persistent endometriosis pain symptoms (i.e., Studies 268 and 270). Each study assessed endometriosis-associated pain over 6 months of treatment and recurrence of symptoms for 12-months post treatment.
Subjects were treated for six months with depo-subQ provera 104 [104 mg given subcutaneously every 3 months (2 injections)] or leuprolide [11.25 mg given subcutaneously every 3 months (2 injections) or 3.75 mg given subcutaneously every month (6 injections)]. Study 268 was conducted in the U.S. and Canada and enrolled 274 subjects (136 subjects received depo-subQ provera 104 and 138 subjects received leuprolide). Study 270 was conducted in South America, Europe, and Asia, and enrolled 299 subjects (153 subjects received depo-subQ provera 104 and 146 subjects received leuprolide).
Reduction in endometriosis pain was evaluated using a modified Biberoglu and Behrman scale that consisted of three patient-reported symptoms (i.e., dysmenorrhea, dyspareunia, and pelvic pain not related to menses) and two signs assessed during pelvic examination (i.e., pelvic tenderness and induration). For each category, a favorable response was defined as improvement of at least 1 unit (severity was assessed on a scale of 0 to 3) relative to baseline score (Figure Q).
| Favorable Response = reduction in severity of symptom or sign of ≥1 point on a scale of 0 to 3, as compared to baseline. |
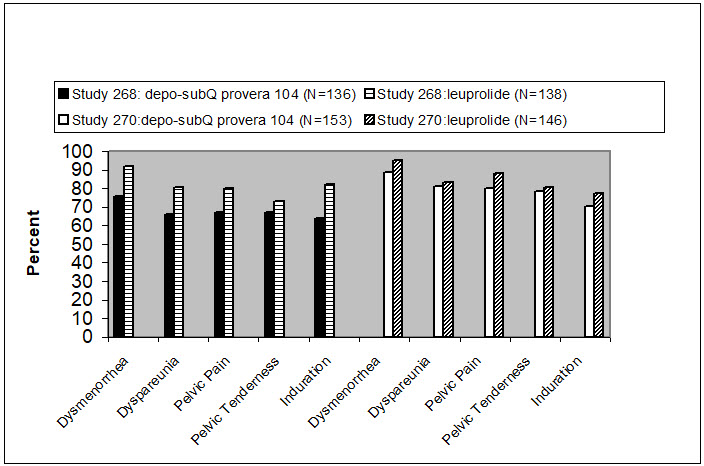 |
Additionally, scores from each of the five categories were combined into a composite score that was considered a global measurement of overall disease improvement. For subjects with baseline scores for each of the 5 categories, a mean decrease of 4 points relative to baseline was considered a clinically meaningful improvement. Across both studies, the mean changes in the composite score met the protocol-defined criterion for improvement for the depo-subQ provera 104 and leuprolide treatment groups.
In the clinical trials, treatment with depo-subQ provera 104 was limited to six months. Data on the persistence of benefit with longer treatment are not available.
14.3 Bone Mineral Density in Women Treated with Depo-medroxyprogesterone acetate for Contraception
In a study that compared changes in bone mineral density (BMD) in adult women using depo-subQ provera 104 or DMPA-IM for contraception, both treatments showed BMD reductions in the lumbar spine, total hip, and femoral neck. Mean percent changes in BMD in depo-subQ provera 104-treated women are shown in Table 3.
| Time on Treatment | Lumbar Spine | Total Hip | Femoral Neck |
|---|---|---|---|
| Mean % Change (95% CI) | Mean % Change (95% CI) | Mean % Change (95% CI) |
|
| 1 year (n=166) | -2.7 (-3.1 to -2.3) | -1.7 (-2.1 to -1.3) | -1.9 (-2.5 to -1.4) |
| 2 years (n=106) | - 4.1 (-4.6 to -3.5) | -3.5 (-4.2 to -2.7) | -3.5 (-4.3 to -2.6) |
BMD Recovery Post-Treatment in Women
Given the similar effects on BMD from depo-subQ provera 104 and DMPA-IM described above, BMD recovery post-treatment is also expected to be similar. In a controlled clinical study that compared changes in BMD in adult women using DMPA-IM for contraception or no hormonal contraception, the 2-year post-treatment follow-up demonstrated incomplete recovery of BMD following the last injection of DMPA-IM. Table 4 shows the change in BMD in women after 5 years of treatment with DMPA-IM and in the control group, as well as the extent of BMD recovery in the subset of women for whom 2-year post-treatment data were available.
| Time in Study | Spine | Total Hip | Femoral Neck | |||
|---|---|---|---|---|---|---|
| DMPA-IM* | Control† | DMPA-IM* | Control† | DMPA-IM* | Control† | |
| 5 years | -5.38% n=33 | 0.43% n=105 | -5.16% n=21 | 0.19% n=65 | -6.12% n=34 | -0.27% n=106 |
| 7 years | -3.13% n=12 | 0.53% n=60 | -1.34% n=7 | 0.94% n=39 | -5.38% n=13 | -0.11% n=63 |
14.4 Bone Mineral Density Changes in Adolescent Females (12 to 18 years of age) Treated with DMPA-IM
The effect of DMPA-IM on BMD in adolescents is described below, and the effect of depo-subQ provera 104 on BMD in adolescents is expected to be similar. The impact of DMPA-IM use for up to 240 weeks (4.6 years) was evaluated in an open-label non-randomized clinical study in 389 adolescent females (12 to 18 years of age). Use of DMPA-IM was associated with a significant decline from baseline in BMD.
Partway through the trial, DMPA-IM administration was stopped (at 120 weeks). The mean number of injections per DMPA-IM user was 9.3. Table 5 summarizes the study findings. The decline in BMD at total hip and femoral neck was greater with longer duration of use. The mean decrease in BMD at 240 weeks was more pronounced at total hip (-6.4%) and femoral neck (-5.4%) compared to lumbar spine (-2.1%).
Adolescents in the untreated cohort had an increase in BMD during the period of growth following menarche. However, the two cohorts were not matched at baseline for age, gynecologic age, race, BMD, and other factors that influence the rate of acquisition of BMD.
| Duration of Treatment | DMPA-IM (150 mg) | Unmatched, Untreated Cohort | ||
|---|---|---|---|---|
| N | Mean % Change | N | Mean % Change | |
| Total Hip BMD | ||||
| Week 60 (1.2 years) | 113 | -2.75 | 166 | 1.22 |
| Week 120 (2.3 years) | 73 | -5.40 | 109 | 2.19 |
| Week 240 (4.6 years) | 28 | -6.40 | 84 | 1.71 |
| Femoral Neck BMD | ||||
| Week 60 | 113 | -2.96 | 166 | 1.75 |
| Week 120 | 73 | -5.30 | 108 | 2.83 |
| Week 240 | 28 | -5.40 | 84 | 1.94 |
| Lumbar Spine BMD | ||||
| Week 60 | 114 | -2.47 | 167 | 3.39 |
| Week 120 | 73 | -2.74 | 109 | 5.28 |
| Week 240 | 27 | -2.11 | 84 | 6.40 |
BMD Recovery Post-Treatment in Adolescents
Longer duration of treatment and smoking were associated with less recovery of BMD following the last injection of DMPA-IM. Table 6 shows the extent of recovery of BMD up to 60 months post-treatment for adolescents who received DMPA-IM for two years or less compared to more than two years. Post-treatment follow-up showed that, in adolescents treated for more than two years, only lumbar spine BMD recovered to baseline levels after treatment was discontinued. Adolescents treated with DMPA-IM for more than two years did not recover to their baseline BMD level at the femoral neck and total hip even up to 60 months post-treatment. Adolescents in the untreated cohort gained BMD throughout the trial period [see Warnings and Precautions (5.1)].
| Duration of Treatment (Months) | 2 Years or Less | More than 2 Years | ||
|---|---|---|---|---|
| N | Mean % Change from baseline | N | Mean % Change from baseline | |
| Total Hip BMD | ||||
| End of Treatment | 49 | -1.5% | 49 | -6.2% |
| 12 M post-treatment | 33 | -1.4% | 24 | -4.6% |
| 24 M post-treatment | 18 | 0.3% | 17 | -3.6% |
| 36 M post-treatment | 12 | 2.1% | 11 | -4.6% |
| 48 M post-treatment | 10 | 1.3% | 9 | -2.5% |
| 60 M post-treatment | 3 | 0.2% | 2 | -1.0% |
| Femoral Neck BMD | ||||
| End of Treatment | 49 | -1.6% | 49 | -5.8% |
| 12 M post-treatment | 33 | -1.4% | 24 | -4.3% |
| 24 M post-treatment | 18 | 0.5% | 17 | -3.8% |
| 36 M post-treatment | 12 | 1.2% | 11 | -3.8% |
| 48 M post-treatment | 10 | 2.0% | 9 | -1.7% |
| 60 M post-treatment | 3 | 1.0% | 2 | -1.9% |
| Lumbar Spine BMD | ||||
| End of Treatment | 49 | -0.9% | 49 | -3.5% |
| 12 M post-treatment | 33 | 0.4% | 23 | -1.1% |
| 24 M post-treatment | 18 | 2.6% | 17 | 1.9% |
| 36 M post-treatment | 12 | 2.4% | 11 | 0.6% |
| 48 M post-treatment | 10 | 6.5% | 9 | 3.5% |
| 60 M post-treatment | 3 | 6.2% | 2 | 5.7% |
14.5 Bone Fracture Incidence in Women Treated with Depo-medroxyprogesterone acetate for Contraception
A retrospective cohort study to assess the association between DMPA-IM injection and the incidence of bone fractures was conducted in 312,395 female contraceptive users in the UK. The incidence rates of fracture were compared between DMPA-IM users and contraceptive users who had no recorded use of DMPA-IM. The Incident Rate Ratio (IRR) for any fracture during the follow-up period (mean=5.5 years) was 1.41 (95% CI 1.35, 1.47). It is not known if this is due to DMPA-IM use or to other related lifestyle factors that have a bearing on fracture rate.
In the study, when cumulative exposure to DMPA-IM was calculated, the fracture rate in users who received fewer than 8 injections was higher than that in women who received 8 or more injections. However, it is not clear that cumulative exposure, which may include periods of intermittent use separated by periods of non-use, is a useful measure of risk, as compared to exposure measures based on continuous use.
There were very few osteoporotic fractures (fracture sites known to be related to low BMD) in the study overall, and the incidence of osteoporotic fractures was not found to be higher in DMPA-IM users compared to non-users.
Importantly, this study could not determine whether use of DMPA-IM has an effect on fracture rate later in life. Given the similar effects on BMD from depo-subQ provera 104 and DMPA-IM described above, bone fracture incidence may also be expected to be similar.
14.6 Bone Mineral Density in Women Treated with Depo-subQ provera 104 for Endometriosis
In two clinical studies of 573 adult women with endometriosis, the BMD effects of 6 months of depo-subQ provera 104 treatment (104 mg subcutaneously every 3 months) were compared to 6 months of leuprolide treatment (either 11.25 mg given subcutaneously every 3 months or 3.75 mg given subcutaneously every month). Subjects were then observed after treatment completion, for an additional 12 months. See Table 7 for the results.
| Time of BMD Measurement | Lumbar Spine | Total Hip | ||||||
|---|---|---|---|---|---|---|---|---|
| depo-subQ provera 104 | Leuprolide | depo-subQ provera 104 | Leuprolide | |||||
| N | Mean % Change | N | Mean % Change | N | Mean % Change | N | Mean % Change | |
| Month 6 of treatment (End of Treatment) | 208 | -1.20 | 229 | -4.10 | 207 | -0.03 | 227 | -1.83 |
| 6 months post-treatment | 168 | -1.06 | 180 | -2.75 | 169 | -0.05 | 181 | -1.59 |
| 12 months post-treatment | 124 | -0.54 | 133 | -1.48 | 125 | 0.39 | 134 | -1.15 |
15. REFERENCES
- Li CI, Beaber EF, Tang MCT et al. Effect of Depo-Medroxyprogesterone Acetate on Breast Cancer Risk among Women 20 to 44 years of Age. Cancer Research 2012; 72:2028-2035.
- Paul C, Skegg DCG, Spears GFS. Depot medroxyprogesterone (Depo-Provera) and risk of breast cancer. Br Med J 1989; 299:759-62.
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Loss of Bone Mineral Density
Advise the patient that the use of depo-subQ provera 104 decreases BMD [see Warnings and Precautions (5.1)].
Arterial and Venous Thromboembolic Disorders
Advise the patient that serious arterial and venous thrombotic events have been seen in women treated with depot medroxyprogesterone acetate (DMPA) [see Warnings and Precautions (5.2)].
Anaphylaxis
Counsel patients on the importance of seeking urgent medical attention if they experience symptoms of anaphylaxis [see Warnings and Precautions (5.5)].
Ectopic Pregnancy
Advise patients to tell their healthcare professional right away if they become pregnant or experience severe abdominal pain to exclude a diagnosis of ectopic pregnancy [see Warnings and Precautions (5.4)].
Bleeding Irregularities
Advise patients at the beginning of treatment that their menstrual cycle may be disrupted, resulting in irregular and unpredictable bleeding or spotting. Explain that bleeding and spotting irregularities usually decrease to the point of amenorrhea as treatment with depo-subQ provera 104 continues, and does not require other therapy [see Warnings and Precautions (5.11)].
Delayed Return of Ovulation and Fertility
Advise patients that return to ovulation and fertility is likely to be delayed after stopping depo-subQ provera 104 [see Warnings and Precautions (5.8)].
Risks of Breast Cancer
Counsel patients about the possible increased risk of breast cancer in women who use depo-subQ provera 104 [see Warnings and Precautions (5.3)].
Depression
Counsel patients about the possible risk of depression and mood disorders. Advise patients with a history of depression or who are receiving treatment for depression to be alert to any mood changes or worsening of their depression. Counsel patients to follow up with their healthcare professional accordingly [see Warnings and Precautions (5.9)].
Risk of Hyperglycemia in Patients with Diabetes
Advise diabetic patients that some patients receiving progestins may exhibit a decrease in glucose tolerance and hyperglycemia [see Warnings and Precautions (5.12)].
Liver Dysfunction
Advise patients to seek medical advice if they experience symptoms of liver problems such as jaundice [see Warnings and Precautions (5.13)].
Fluid Retention
Counsel patients with conditions that may be influenced by fluid retention to inform their healthcare professional if they experience symptoms of fluid retention [see Warnings and Precautions (5.6)].
Injection Site Reactions
Counsel patients on injection site reactions. Advise patients that dimpling or scarring may occur in 1 out of 100 patients [see Warnings and Precautions (5.10)].
Sexually Transmitted Infections
Counsel patients that depo-subQ provera 104 does not protect against HIV infection (AIDS) and other sexually transmitted infections [see Warnings and Precautions (5.14)].
Drug Interactions
Counsel patients to contact their healthcare professional if they start a medication that is a CYP3A enzyme inducer [see Drug Interactions (7)]. Advise patients that taking a medication that is a CYP3A enzyme inducer may require using a back-up or alternate contraceptive method.
This product's labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com.

LAB-0295-16.0
PATIENT INFORMATION
DEPO-SUBQ PROVERA 104®
(deh-poh' sub-cue' pro-ver-ah' one-oh-four)
(medroxyprogesterone acetate injectable suspension)
for subcutaneous use
WHAT IS THE MOST IMPORTANT INFORMATION I SHOULD KNOW ABOUT depo-subQ provera 104?
Use of depo-subQ provera 104 may cause you to lose calcium stored in your bones. The longer you use depo-subQ provera 104 the more calcium you are likely to lose. The calcium may not return completely once you stop using depo-subQ provera 104.
Loss of calcium may cause weak, porous bones (osteoporosis) that could increase the risk that your bones might break, especially after menopause. It is not known whether your risk of developing osteoporosis may be greater if you are a teenager when you start to use depo-subQ provera 104.
You should use depo-subQ provera 104 long-term (for example, more than 2 years) only if other methods of birth control or other treatments for endometriosis pain are not right for you.
Depo-subQ provera 104 does not protect you from HIV (AIDS) and other infections spread through sex (STIs).
WHAT IS depo-subQ provera 104?
Depo-subQ provera 104 is a drug for birth control. It also helps relieve pain related to endometriosis (en-do-ME-tree-OH-sis). Symptoms of endometriosis arise when cells normally inside your uterus grow outside the uterus. The cells respond to menstrual cycle hormones, and may cause painful periods, pelvic pain, and painful sex.
Depo-subQ provera 104 contains a hormone called medroxyprogesterone acetate (MPA). It is given as a shot (injection) every 3 months. Three months is the same as 12 to 14 weeks.
HOW WELL DOES depo-subQ provera 104 WORK FOR PREVENTING PREGNANCY?
When you use depo-subQ provera 104 correctly, the chance of getting pregnant is very low. In studies, no women became pregnant during the year they used depo-subQ provera 104 injection.
The list below estimates the chances of getting pregnant using different types of birth control. The numbers are based on typical use. Typical use includes people who use the method correctly and people who use the method incorrectly. The list shows the number of women out of 100 women who will likely get pregnant if they use the method for 1 year.
| Method | Typical Chance of Getting Pregnant in 1 year (Number of pregnancies in 100 women) |
|---|---|
| Shot Implant Female sterilization Male sterilization IUD (copper IUD and levonorgestrel IUD) | Less than 1 |
| Pill | 5 |
| Condom alone (male) | 14 |
| Withdrawal | 19 |
| Diaphragm with spermicides | 20 |
| Condom alone (female) | 21 |
| Periodic abstinence | 25 |
| Spermicides alone | 26 |
| Vaginal sponge or Cervical cap with spermicide | 20 to 40 |
HOW WILL I GET depo-subQ provera 104?
Depo-subQ provera 104 is given as a shot just under the skin on your thigh or belly. You get it once every 3 months.
For Birth Control
First shot:
Your healthcare professional will want to be sure that you are not pregnant before you get your first shot. Normally, you get the shot by the 5th day from the START of your menstrual period. You get it whether or not you are still bleeding.
If you are breast-feeding, you may have your first shot as early as 6 weeks after you deliver your baby.
After the first shot:
It is very important to keep getting depo-subQ provera 104 every 3 months. If you wait more than 14 weeks between shots, you could become pregnant. Your healthcare professional must make sure you are not pregnant before you get your next shot.
When you get your shot, make an appointment for your next shot. Mark it on your calendar.
If you need a birth control method for more than two years, your healthcare professional may ask you to have a test of your bones or ask you to switch to another birth control method before continuing depo-subQ provera 104, especially if you have other risks for weak bones.
For Endometriosis
If you have regular periods, you will get depo-subQ provera 104 the same way as described above for birth control. If your periods have stopped or are not regular, your healthcare professional must test to make sure you are not pregnant before you get your first shot.
It is not recommended that you receive depo-subQ provera 104 for treatment of endometriosis for longer than 2 years. If your painful symptoms return after stopping treatment, your healthcare professional should ask you to have a test of your bones before restarting treatment.
WHAT IF I MISS A SHOT?
If you miss a shot, or wait longer than 14 weeks between shots, you could get pregnant. The longer you wait, the greater the risk of getting pregnant.
Talk with your healthcare professional to find out when to restart depo-subQ provera 104. You should be tested to be sure you are not pregnant.
Use another kind of non-hormonal birth control, such as condoms, until you start depo-subQ provera 104 again.
DO NOT TAKE depo-subQ provera 104 IF YOU…
- Have any unexplained vaginal bleeding
- Have or have ever had breast cancer or think you have breast cancer
- Ever had serious blood clots, such as blood clots in your legs (deep venous thrombophlebitis), lungs (pulmonary embolism), heart (heart attack), or head (stroke)
- Have liver disease
- Are allergic to anything in depo-subQ provera 104. There is a list of what is in depo-subQ provera 104 at the end of this leaflet.
BEFORE TAKING depo-subQ provera 104
Your healthcare professional may do a physical examination and check your blood and urine.
Tell your healthcare professional about all your medical conditions.
Most importantly, tell your healthcare professional if you:
- Are pregnant or might be pregnant. You should not get depo-subQ provera 104 if you are pregnant.
- Plan to become pregnant in the next year. After you stop getting depo-subQ provera 104, it takes time for your body to be able to get pregnant. It can be as early as 1 week after the last shot wears off. Most likely it will take up to 1 year or longer for you to get pregnant.
- Have or have ever had breast cancer, or think you have breast cancer
- Have breast cancer in your family
- Have an abnormal mammogram (breast X-ray), lumps in your breast, or bleeding from your nipples
- Have irregular, light, or heavy menstrual periods
- Have or had any of the following medical problems:
- Kidney problems
- High blood pressure
- Migraine headaches
- Asthma
- Seizures
- Diabetes, or if it runs in your family
- Depression
- Heart attack, stroke, or blood clots
- Bone disease
- Anorexia nervosa (an eating disorder)
- A strong family history of osteoporosis
- Use of a drug that can lower the amount of calcium in bones (drugs for epilepsy or steroids)
- Drinking a lot of alcohol or smoking a lot
It is important to see your healthcare professional regularly if you have any of these conditions.
Some medicines may make depo-subQ provera 104 less effective at preventing pregnancy, including those listed below:
- Bosentan (used to treat pulmonary arterial hypertension)
- Efavirenz, etravirine (HIV medicines)
- Modafinil (used to improve wakefulness)
- Mitotane (used to treat adrenal cortical carcinoma)
- Phenytoin, carbamazepine, phenobarbital (used to treat seizures)
- Rifampin (an antibiotic)
- St. John's Wort (herbal medicinal product)
Tell your healthcare professional about all the medicines you take. This includes prescription and over-the-counter medicines, vitamins, and herbal products.
WHAT ELSE SHOULD I KNOW ABOUT TAKING depo-subQ provera 104?
Other Birth Control. If you can't take birth control pills or can't use a birth control patch or ring, you may be able to use depo-subQ provera 104. Ask your healthcare professional.
Pregnancy. When you take depo-subQ provera 104 every 3 months, your chance of getting pregnant is very low. You could miss a period or have a light period and not be pregnant. If you miss 1 or 2 periods and think you might be pregnant, see your healthcare professional as soon as possible.
You should not use depo-subQ provera 104 if you are pregnant. However, depo-subQ provera 104 taken by accident during pregnancy does not seem to cause birth defects.
Pregnancy in your fallopian tubes (Ectopic Pregnancy). If you have severe pain low in your belly, tell your healthcare professional right away. Infrequently, a baby may start to grow outside the uterus, most often in the tubes.
Nursing a baby. Wait at least 6 weeks after your baby is born to start depo-subQ provera 104. You can use depo-subQ provera 104 if you are nursing.
It does not lower the amount of milk you can make.
It can pass through breast milk into your baby, but it is not harmful.
Blood or urine tests. Depo-subQ provera 104 may affect blood or urine test results. Tell your healthcare professional you are taking depo-subQ provera 104 if you are going to have blood or urine tests.
Liver problems. Your healthcare professional may stop depo-subQ provera 104 if you have liver problems. Some signs of liver problems are yellow skin or eyes, feeling like you have the flu, feeling more tired than usual, and itching. Tell your healthcare professional if you have these symptoms.
WHAT ARE THE MOST SERIOUS RISKS OF depo-subQ provera 104?
Losing calcium from your bones. Depo-subQ provera 104 use may decrease the amount of calcium in your bones. The longer you use depo-subQ provera 104, the more calcium you are likely to lose. This increases the risk of your bones weakening if you use depo-subQ provera 104 continuously for a long time (for example, if you use depo-subQ provera 104 for more than 2 years). The loss of calcium may increase your risk of osteoporosis and broken bones, particularly after your menopause.
Calcium is generally added to the bones during teenage years. The decrease of calcium in your bones is of most concern if you are a teenager or have the following risk factors:
- Bone disease
- Anorexia nervosa (an eating disorder)
- A strong family history of osteoporosis
- Using a drug that can lower the amount of calcium in bones (drugs for epilepsy or steroids), or
- Drinking a lot of alcohol or smoking a lot
If you need a birth control method for more than 2 years, your healthcare professional may ask you to have a test of your bones or ask you to switch to another birth control method before continuing depo-subQ provera 104, especially if you have other risks for weak bones. When depo-subQ provera 104 is stopped, the calcium in your bones begins to come back. The lost calcium may not return completely once you stop using depo-subQ provera 104.
Abnormal or very heavy bleeding. If you start having very heavy or very long periods, tell your healthcare professional.
Allergic reaction. Allergic reactions to depo-subQ provera 104 have been reported. If you have hives, problems breathing, swelling of the face, mouth, tongue, or neck, or just do not feel right after your shot, call your healthcare professional or go to the Emergency Room right away.
Serious blood clots. Call your healthcare professional immediately if you:
- Have sharp chest pain, cough blood, or suddenly have trouble breathing
- Have a sudden severe headache with vomiting, blindness or trouble talking, weakness, or numbness in an arm or leg, or get dizzy or faint
- Have swelling or severe pain in your leg
Depression. If you suffer from depression or have a history of depression, inform your healthcare provider if you notice any worsening of your depression while taking depo-subQ provera 104.
WHAT ARE COMMON SIDE EFFECTS OF depo-subQ provera 104?
The most common side effects are:
- Changes in your monthly periods. You may not know when you will bleed, your periods may not be regular, you may have heavy bleeding, or you may have spotting. You may have more days of bleeding during the first 2 or 3 months after you start depo-subQ provera 104. Over time, you may have less and less bleeding. Many women stop having periods by the end of 1 year. Your periods will come back eventually after you stop using depo-subQ provera 104.
- Headache.
- Weight gain. In studies, women gained an average of 3 to 4 pounds during the first year they used depo-subQ provera 104. After 2 years of using depo-subQ provera 104, women gained an average of 7 to 8 pounds. Some women gained more, some gained less, some lost, and some stayed the same. Weight changes beyond 2 years of use with depo-subQ provera 104 have not been studied. Women who used a similar birth control product for 5 years gained on average 5 pounds more than women who did not use a hormone contraceptive product.
- Skin reaction where you got the shot. Lumps, skin dimpling, or pain are usually mild and usually don't last long. Scarring is uncommon, but may happen. If there is swelling or your skin gets hot, has pus or looks bruised 1 or more days after your shot, call your healthcare professional.
Women using depo-subQ provera 104 for birth control or endometriosis had these less common side effects: Vaginal inflammation, vaginal thrush, abdominal pain, urinary tract infections, acne, depression, less sex drive, nausea, back pain, breast pain/tenderness, fatigue, anxiety, being irritable, dizziness, hot flushes and fluid retention.
If you feel you are having other side effects, talk with your healthcare professional.
DOES depo-subQ provera 104 CAUSE CANCER?
There have been several studies of women who use birth control like depo-subQ provera 104.
- Women who use depo-subQ provera 104 may have a slightly increased risk of breast cancer compared to non-users.
- The risk of cancer of the ovary, liver, or cervix did not change.
WHAT IF I WANT TO BECOME PREGNANT?
Plan ahead. The effect of depo-subQ provera 104 can last for a long time after you stop getting shots. Although you may be able to get pregnant quickly, it is more likely to take a year or longer after your last shot before you get pregnant.
It's best to see your healthcare professional for a pre-pregnancy check-up. Your healthcare professional may also tell you to take a vitamin called folic acid every day if you are planning to become pregnant.
GENERAL ADVICE ABOUT depo-subQ provera 104
For more information about depo-subQ provera 104, ask your healthcare professional or pharmacist. You can also visit www.depo-subQprovera104.com or call 1-866-554 DEPO (3376). A nurse can answer questions in Spanish or English 24 hours-a-day, 7 days a week.
WHAT IS IN depo-subQ provera 104?
Active ingredient: medroxyprogesterone acetate.
Inactive ingredients: methylparaben, propylparaben, sodium chloride, polyethylene glycol, polysorbate 80, monobasic sodium phosphate∙H2O, dibasic sodium phosphate∙12H2O, methionine, povidone, water for shot. When necessary, the pH is adjusted with sodium hydroxide or hydrochloric acid, or both.
This product's labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com.

LAB-0298-9.0
Revised December 2020
PRINCIPAL DISPLAY PANEL - 0.65 mL Syringe Label
PAA142059
depo-subQ
provera 104®
(medroxyprogesterone acetate) injectable
suspension (104 mg/0.65 mL for subcutaneous use)
104 mg/0.65 mL
0.65 mL Single Dose Syringe
Store at 20°C to 25°C (68°F to 77°F).
Do not refrigerate.
Shake vigorously before use.
Pharmacia & Upjohn Co
LOT/EXP:

PRINCIPAL DISPLAY PANEL - 0.65 mL Syringe Carton
NDC 0009-4709-13
Single Dose 0.65 mL Prefilled Syringe
depo-subQ provera 104®
(medroxyprogesterone acetate) injectable suspension
(104 mg/0.65 mL for subcutaneous use)
104 mg/0.65 mL
Pfizer
Distributed by
Pharmacia & Upjohn Co
Division of Pfizer Inc, NY, NY 10017
Rx only
