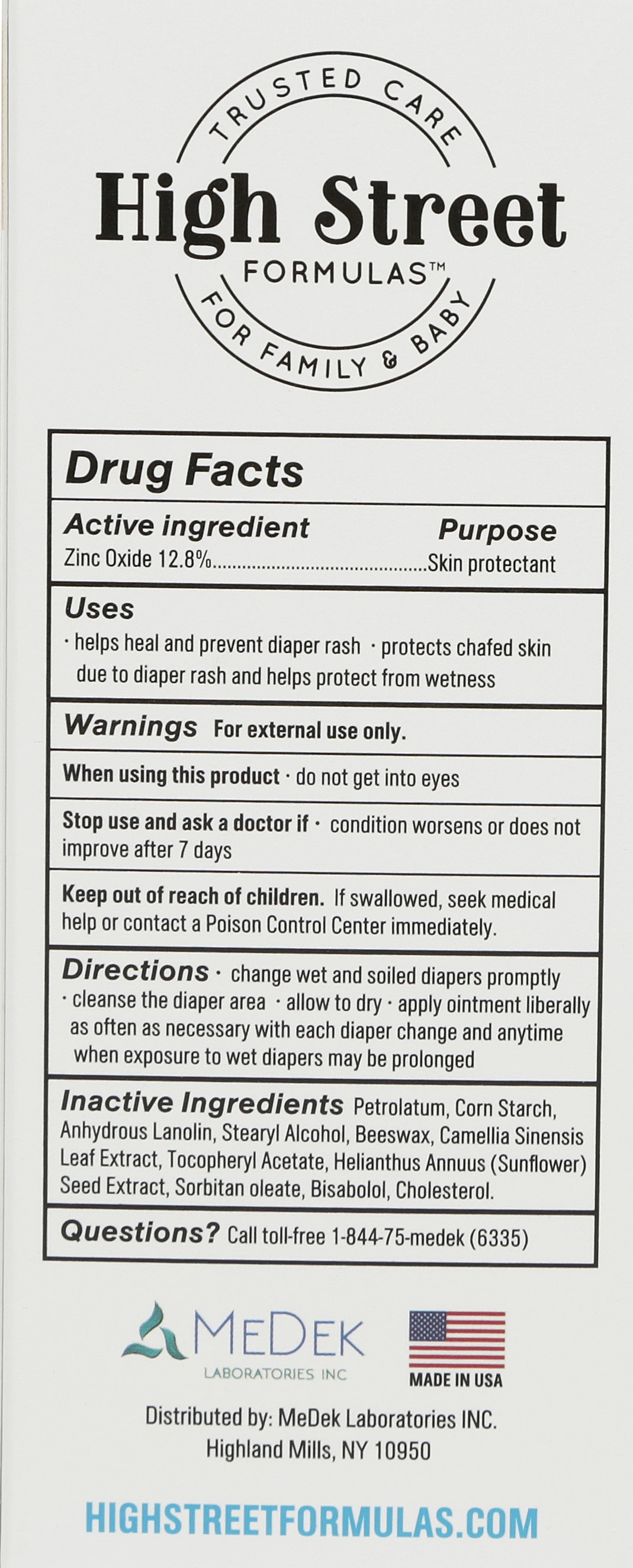
DIAPER- zinc oxide ointment
Medek Laboratories Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredients Purpose
Zinc Oxide 12.8%..............................................................Skin Protectant
Warnings
FOR EXTERNAL USE ONLY
When using this product
When using this product keep out of eyes.
Stop use and ask a doctor if
Stop use and ask a doctor if condition worsens or does not improve after 7 days
Keep out of reach of children
Keep out of reach of children.
Other Information
protect this product from excessive heat and direct sun
Inactive Ingredients
Petrolatum, corn starch, anhydrous lanolin, stearyl alcohol, beeswax, camellia sinensis leaf extract, tocopheryl acetate, sunflower seed extract, sorbitan oleate, bisabolol, cholesterol
Indications
Uses
Help heal and prevent diaper rash
Protects chafed skin due to diaper rash and helps protect wetness
Do Not Use
Discontinue use if irritation occurs.
Ask a doctor
Ask a doctor if condition worsens
Directions
change wet and soiled diapers promptly
cleanse the diaper area
allow to dry
apply ointment liberally as often as necessary with each diaper change and anytime when exposure to wet diapers may be prolonged
dosage
apply ointment liberally as often as necessary with each diaper change and anytime when exposure to wet diapers may be prolonged
image of label
Medek Laboratories Inc.
