FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Alogliptin tablets are indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus [see Clinical Studies (14)].
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
The recommended dose of alogliptin tablets is 25 mg once daily. Alogliptin tablets may be taken with or without food. Do not split tablets.
2.2 Patients with Renal Impairment
No dose adjustment of alogliptin tablets is necessary for patients with mild renal impairment (creatinine clearance [CrCl] ≥60 mL/min).
The dose of alogliptin tablets is 12.5 mg once daily for patients with moderate renal impairment (CrCl ≥30 to <60 mL/min).
The dose of alogliptin tablets is 6.25 mg once daily for patients with severe renal impairment (CrCl ≥15 to <30 mL/min) or with end-stage renal disease (ESRD) (CrCl <15 mL/min or requiring hemodialysis). Alogliptin tablets may be administered without regard to the timing of dialysis. Alogliptin tablets have not been studied in patients undergoing peritoneal dialysis [see Use in Specific Populations (8.6), Clinical Pharmacology (12.3)].
Because there is a need for dose adjustment based upon renal function, assessment of renal function is recommended prior to initiation of alogliptin tablets therapy and periodically thereafter.
3 DOSAGE FORMS AND STRENGTHS
- 25 mg tablets are light red, oval, biconvex, film-coated, with "TAK ALG-25" printed on one side.
- 12.5 mg tablets are yellow, oval, biconvex, film-coated, with "TAK ALG-12.5" printed on one side.
- 6.25 mg tablets are light pink, oval, biconvex, film-coated, with "TAK ALG-6.25" printed on one side.
4 CONTRAINDICATIONS
Serious hypersensitivity reaction to alogliptin or any of the excipients in Alogliptin tablets. Reactions such as anaphylaxis, angioedema and severe cutaneous adverse reactions have been reported [see Warnings and Precautions (5.3), Adverse Reactions (6.2)].
5 WARNINGS AND PRECAUTIONS
5.1 Pancreatitis
Acute pancreatitis has been reported in the postmarketing setting and in randomized clinical trials. In glycemic control trials in patients with type 2 diabetes, acute pancreatitis was reported in 6 (0.2%) patients treated with alogliptin tablets 25 mg and 2 (<0.1%) patients treated with active comparators or placebo. In the EXAMINE trial (a cardiovascular outcomes trial of patients with type 2 diabetes and high cardiovascular (CV) risk), acute pancreatitis was reported in 10 (0.4%) of patients treated with alogliptin tablets and in 7 (0.3%) of patients treated with placebo.
It is unknown whether patients with a history of pancreatitis are at increased risk for pancreatitis while using alogliptin tablets.
After initiation of alogliptin tablets, patients should be observed for signs and symptoms of pancreatitis. If pancreatitis is suspected, alogliptin tablets should promptly be discontinued and appropriate management should be initiated.
5.2 Heart Failure
In the EXAMINE trial which enrolled patients with type 2 diabetes and recent acute coronary syndrome, 106 (3.9%) of patients treated with alogliptin tablets and 89 (3.3%) of patients treated with placebo were hospitalized for congestive heart failure.
Consider the risks and benefits of alogliptin tablets prior to initiating treatment in patients at risk for heart failure, such as those with a prior history of heart failure and a history of renal impairment, and observe these patients for signs and symptoms of heart failure during therapy. Patients should be advised of the characteristic symptoms of heart failure and should be instructed to immediately report such symptoms. If heart failure develops, evaluate and manage according to current standards of care and consider discontinuation of alogliptin tablets.
5.3 Hypersensitivity Reactions
There have been postmarketing reports of serious hypersensitivity reactions in patients treated with alogliptin tablets. These reactions include anaphylaxis, angioedema and severe cutaneous adverse reactions, including Stevens-Johnson syndrome. If a serious hypersensitivity reaction is suspected, discontinue alogliptin tablets, assess for other potential causes for the event and institute alternative treatment for diabetes [see Adverse Reactions (6.2)]. Use caution in patients with a history of angioedema with another dipeptidyl peptidase-4 (DPP-4) inhibitor because it is unknown whether such patients will be predisposed to angioedema with alogliptin tablets.
5.4 Hepatic Effects
There have been postmarketing reports of fatal and nonfatal hepatic failure in patients taking alogliptin tablets, although some of the reports contain insufficient information necessary to establish the probable cause [see Adverse Reactions (6.2)].
In glycemic control trials in patients with type 2 diabetes, serum alanine aminotransferase (ALT) elevations greater than three times the upper limit of normal (ULN) were reported in 1.3% of patients treated with alogliptin tablets 25 mg and 1.7% of patients treated with active comparators or placebo. In the EXAMINE trial (a cardiovascular outcomes trial of patients with type 2 diabetes and high cardiovascular (CV) risk), increases in serum alanine aminotransferase three times the upper limit of the reference range occurred in 2.4% of patients treated with alogliptin tablets and in 1.8% of patients treated with placebo.
Measure liver tests promptly in patients who report symptoms that may indicate liver injury, including fatigue, anorexia, right upper abdominal discomfort, dark urine or jaundice. In this clinical context, if the patient is found to have clinically significant liver enzyme elevations and if abnormal liver tests persist or worsen, alogliptin tablets should be interrupted and investigation done to establish the probable cause. Alogliptin tablets should not be restarted in these patients without another explanation for the liver test abnormalities.
5.5 Hypoglycemia with Concomitant Use with Insulin or Insulin Secretagogues
Insulin and insulin secretagogues, such as sulfonylureas, are known to cause hypoglycemia. Therefore, a lower dose of insulin or insulin secretagogue may be required to minimize the risk of hypoglycemia when used in combination with alogliptin tablets.
5.6 Severe and Disabling Arthralgia
There have been postmarketing reports of severe and disabling arthralgia in patients taking DPP-4 inhibitors. The time to onset of symptoms following initiation of drug therapy varied from one day to years. Patients experienced relief of symptoms upon discontinuation of the medication. A subset of patients experienced a recurrence of symptoms when restarting the same drug or a different DPP-4 inhibitor. Consider DPP-4 inhibitors as a possible cause for severe joint pain and discontinue drug if appropriate.
5.7 Bullous Pemphigoid
Postmarketing cases of bullous pemphigoid requiring hospitalization have been reported with DPP-4 inhibitor use. In reported cases, patients typically recovered with topical or systemic immunosuppressive treatment and discontinuation of the DPP-4 inhibitor. Tell patients to report development of blisters or erosions while receiving alogliptin tablets. If bullous pemphigoid is suspected, alogliptin tablets should be discontinued and referral to a dermatologist should be considered for diagnosis and appropriate treatment.
6 ADVERSE REACTIONS
The following serious adverse reactions are described below or elsewhere in the prescribing information:
- Pancreatitis [see Warnings and Precautions (5.1)]
- Heart Failure [see Warnings and Precautions (5.2)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.3)]
- Hepatic Effects [see Warnings and Precautions (5.4)]
- Severe and Disabling Arthralgia [see Warnings and Precautions (5.6)]
- Bullous Pemphigoid [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
A total of 14,778 patients with type 2 diabetes participated in 14 randomized, double-blind, controlled clinical trials of whom 9052 subjects were treated with alogliptin tablets, 3469 subjects were treated with placebo and 2257 were treated with an active comparator. The mean duration of diabetes was seven years, the mean body mass index (BMI) was 31 kg/m2 (49% of patients had a BMI ≥30 kg/m2), and the mean age was 58 years (26% of patients ≥65 years of age). The mean exposure to alogliptin tablets was 49 weeks with 3348 subjects treated for more than one year.
In a pooled analysis of these 14 controlled clinical trials, the overall incidence of adverse reactions was 73% in patients treated with alogliptin tablets 25 mg compared to 75% with placebo and 70% with active comparator. Overall discontinuation of therapy due to adverse reactions was 6.8% with alogliptin tablets 25 mg compared to 8.4% with placebo or 6.2% with active comparator.
Adverse reactions reported in ≥4% of patients treated with alogliptin tablets 25 mg and more frequently than in patients who received placebo are summarized in Table 1.
| Number of Patients (%) | |||
|---|---|---|---|
| Alogliptin Tablets 25 mg | Placebo | Active Comparator | |
| N=6447 | N=3469 | N=2257 | |
| Nasopharyngitis | 309 (4.8) | 152 (4.4) | 113 (5.0) |
| Upper Respiratory Tract Infection | 287 (4.5) | 121 (3.5) | 113 (5.0) |
| Headache | 278 (4.3) | 101 (2.9) | 121 (5.4) |
Hypoglycemia
Hypoglycemic events were documented based upon a blood glucose value and/or clinical signs and symptoms of hypoglycemia.
In the monotherapy study, the incidence of hypoglycemia was 1.5% in patients treated with alogliptin tablets compared to 1.6% with placebo. The use of alogliptin tablets as add-on therapy to glyburide or insulin did not increase the incidence of hypoglycemia compared to placebo. In a monotherapy study comparing alogliptin tablets to a sulfonylurea in elderly patients, the incidence of hypoglycemia was 5.4% with alogliptin tablets compared to 26% with glipizide (Table 2).
|
||
| Add-On to Glyburide (26 Weeks) | Alogliptin Tablets 25 mg | Placebo |
| N=198 | N=99 | |
| Overall (%) | 19 (9.6) | 11 (11.1) |
| Severe (%)† | 0 | 1 (1) |
| Add-On to Insulin (± Metformin) (26 Weeks) | Alogliptin Tablets 25 mg | Placebo |
| N=129 | N=129 | |
| Overall (%) | 35 (27) | 31 (24) |
| Severe (%)† | 1 (0.8) | 2 (1.6) |
| Add-On to Metformin (26 Weeks) | Alogliptin Tablets 25 mg | Placebo |
| N=207 | N=104 | |
| Overall (%) | 0 | 3 (2.9) |
| Severe (%)† | 0 | 0 |
| Add-On to Pioglitazone (± Metformin or Sulfonylurea) (26 Weeks) | Alogliptin Tablets 25 mg | Placebo |
| N=199 | N=97 | |
| Overall (%) | 14 (7.0) | 5 (5.2) |
| Severe (%)† | 0 | 1 (1) |
| Compared to Glipizide (52 Weeks) | Alogliptin Tablets 25 mg | Glipizide |
| N=222 | N=219 | |
| Overall (%) | 12 (5.4) | 57 (26) |
| Severe (%)† | 0 | 3 (1.4) |
| Compared to Metformin (26 Weeks) | Alogliptin Tablets 25 mg | Metformin 500 mg twice daily |
| N=112 | N=109 | |
| Overall (%) | 2 (1.8) | 2 (1.8) |
| Severe (%)† | 0 | 0 |
| Add-On to Metformin Compared to Glipizide (52 Weeks) | Alogliptin Tablets 25 mg | Glipizide |
| N=877 | N=869 | |
| Overall (%) | 12 (1.4) | 207 (23.8) |
| Severe (%)† | 0 | 4 (0.5) |
In the EXAMINE trial, the incidence of investigator reported hypoglycemia was 6.7% in patients receiving alogliptin tablets and 6.5% in patients receiving placebo. Serious adverse reactions of hypoglycemia were reported in 0.8% of patients treated with alogliptin tablets and in 0.6% of patients treated with placebo.
Renal Impairment
In glycemic control trials in patients with type 2 diabetes, 3.4% of patients treated with alogliptin tablets and 1.3% of patients treated with placebo had renal function adverse reactions. The most commonly reported adverse reactions were renal impairment (0.5% for alogliptin tablets and 0.1% for active comparators or placebo), decreased creatinine clearance (1.6% for alogliptin tablets and 0.5% for active comparators or placebo) and increased blood creatinine (0.5% for alogliptin tablets and 0.3% for active comparators or placebo) [see Use in Specific Populations (8.6)].
In the EXAMINE trial of high CV risk type 2 diabetes patients, 23% of patients treated with alogliptin tablets and 21% of patients treated with placebo had an investigator reported renal impairment adverse reaction. The most commonly reported adverse reactions were renal impairment (7.7% for alogliptin tablets and 6.7% for placebo), decreased glomerular filtration rate (4.9% for alogliptin tablets and 4.3% for placebo) and decreased renal clearance (2.2% for alogliptin tablets and 1.8% for placebo). Laboratory measures of renal function were also assessed. Estimated glomerular filtration rate decreased by 25% or more in 21.1% of patients treated with alogliptin tablets and 18.7% of patients treated with placebo. Worsening of chronic kidney disease stage was seen in 16.8% of patients treated with alogliptin tablets and in 15.5% of patients treated with placebo.
6.2 Postmarketing Experience
The following adverse reactions have been identified during the postmarketing use of alogliptin tablets. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Gastrointestinal disorders: acute pancreatitis, diarrhea, constipation, nausea, ileus
Hepatobiliary disorders: fulminant hepatic failure
Immune system disorders: hypersensitivity reactions including anaphylaxis
Investigations: hepatic enzyme elevations
Musculoskeletal and Connective Tissue Disorders: severe and disabling arthralgia, rhabdomyolysis
Renal and urinary disorders: tubulointerstitial nephritis
Skin and subcutaneous tissue disorders: angioedema, rash, urticaria and severe cutaneous adverse reactions including Stevens-Johnson syndrome, bullous pemphigoid
7 DRUG INTERACTIONS
Alogliptin tablets are primarily renally excreted. Cytochrome (CYP) P450-related metabolism is negligible. No significant drug-drug interactions were observed with the CYP-substrates or inhibitors tested or with renally excreted drugs [see Clinical Pharmacology (12.3)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Limited data with alogliptin in pregnant women are not sufficient to determine a drug-associated risk for major birth defects or miscarriage. There are risks to the mother and fetus associated with poorly controlled diabetes in pregnancy [see Clinical Considerations].
No adverse developmental effects were observed when alogliptin was administered to pregnant rats and rabbits during organogenesis at exposures 180- and 149-times the 25 mg clinical dose, respectively, based on plasma drug exposure (AUC) [see Data].
The estimated background risk of major birth defects is 6-10% in women with pre-gestational diabetes with a HbA1c >7 and has been reported to be as high as 20-25% in women with HbA1c >10. The estimated background risk of miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Disease-associated maternal and/or embryo/fetal risk
Poorly controlled diabetes in pregnancy increases the maternal risk for diabetic ketoacidosis, pre-eclampsia, spontaneous abortions, preterm delivery and delivery complications. Poorly controlled diabetes increases the fetal risk for major birth defects, still birth, and macrosomia related morbidity.
Animal Data
Alogliptin administered to pregnant rabbits and rats during the period of organogenesis did not cause adverse developmental effects at doses of up to 200 mg/kg and 500 mg/kg, or 149 times and 180 times, the 25 mg clinical dose, respectively, based on plasma drug exposure (AUC). Placental transfer of alogliptin into the fetus was observed following oral dosing to pregnant rats.
No adverse developmental outcomes were observed in offspring when alogliptin was administered to pregnant rats during gestation and lactation at doses up to 250 mg/kg (~ 95 times the 25 mg clinical dose, based on AUC).
8.2 Lactation
Risk Summary
There is no information regarding the presence of alogliptin in human milk, the effects on the breastfed infant, or the effects on milk production. Alogliptin is present in rat milk: however, due to species specific differences in lactation physiology, animal lactation data may not reliably predict levels in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for alogliptin tablets and any potential adverse effects on the breastfed infant from alogliptin tablets or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness of alogliptin tablets in pediatric patients have not been established.
8.5 Geriatric Use
Of the total number of patients (N=9052) in clinical safety and efficacy studies treated with alogliptin tablets, 2257 (24.9%) patients were 65 years and older and 386 (4.3%) patients were 75 years and older. No overall differences in safety or effectiveness were observed between patients 65 years and over and younger patients. While this clinical experience has not identified differences in responses between the elderly and younger patients, greater sensitivity of some older individuals cannot be ruled out.
8.6 Renal Impairment
A total of 602 patients with moderate renal impairment (eGFR ≥30 and <60 mL/min/1.73 m2) and 4 patients with severe renal impairment/end-stage renal disease (eGFR <30 mL/min/1.73 m2 or <15 mL/min/1.73 m2, respectively) at baseline were treated with alogliptin tablets in clinical trials in patients with type 2 diabetes. Reductions in HbA1c were generally similar in this subgroup of patients. The overall incidence of adverse reactions was generally balanced between alogliptin tablets and placebo treatments in this subgroup of patients.
In the EXAMINE trial of high CV risk type 2 diabetes patients, 694 patients had moderate renal impairment and 78 patients had severe renal impairment or end-stage renal disease at baseline. The overall incidences of adverse reactions, serious adverse reactions and adverse reactions leading to study drug discontinuation were generally similar between the treatment groups.
8.7 Hepatic Impairment
No dose adjustments are required in patients with mild to moderate hepatic impairment (Child-Pugh Grade A and B) based on insignificant change in systemic exposures (e.g., AUC) compared to subjects with normal hepatic function in a pharmacokinetic study. Alogliptin tablets have not been studied in patients with severe hepatic impairment (Child-Pugh Grade C). Use caution when administering alogliptin tablets to patients with liver disease [see Warnings and Precautions (5.4)].
10 OVERDOSAGE
In the event of an overdose, it is reasonable to institute the necessary clinical monitoring and supportive therapy as dictated by the patient's clinical status. Per clinical judgment, it may be reasonable to initiate removal of unabsorbed material from the gastrointestinal tract.
Alogliptin is minimally dialyzable; over a three-hour hemodialysis session, approximately 7% of the drug was removed. Therefore, hemodialysis is unlikely to be beneficial in an overdose situation. It is not known if alogliptin tablets are dialyzable by peritoneal dialysis.
To contact the poison control center, call 1-800-222-1222.
11 DESCRIPTION
Alogliptin tablets contain the active ingredient alogliptin, which is a selective, orally bioavailable inhibitor of the enzymatic activity of dipeptidyl peptidase-4 (DPP-4).
Chemically, alogliptin is prepared as a benzoate salt, which is identified as 2-({6-[(3R)-3-aminopiperidin-1-yl]-3-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl}methyl)benzonitrile monobenzoate. It has a molecular formula of C18H21N5O2∙C7H6O2 and a molecular weight of 461.51 daltons. The structural formula is:
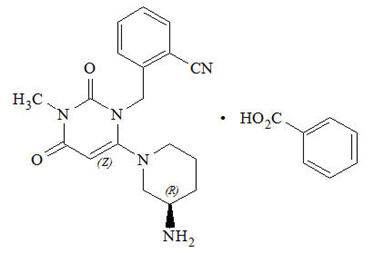
Alogliptin benzoate is a white to off-white crystalline powder containing one asymmetric carbon in the aminopiperidine moiety. It is soluble in dimethylsulfoxide, sparingly soluble in water and methanol, slightly soluble in ethanol and very slightly soluble in octanol and isopropyl acetate.
Each alogliptin tablet contains 34 mg, 17 mg or 8.5 mg alogliptin benzoate, which is equivalent to 25 mg, 12.5 mg or 6.25 mg, respectively, of alogliptin and the following inactive ingredients: mannitol, microcrystalline cellulose, hydroxypropyl cellulose, croscarmellose sodium and magnesium stearate. In addition, the film coating contains the following inactive ingredients: hypromellose, titanium dioxide, ferric oxide (red or yellow) and polyethylene glycol, and is marked with printing ink (Gray F1).
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Increased concentrations of the incretin hormones such as glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are released into the bloodstream from the small intestine in response to meals. These hormones cause insulin release from the pancreatic beta cells in a glucose-dependent manner but are inactivated by the dipeptidyl peptidase-4 (DPP-4) enzyme within minutes. GLP-1 also lowers glucagon secretion from pancreatic alpha cells, reducing hepatic glucose production. In patients with type 2 diabetes, concentrations of GLP-1 are reduced but the insulin response to GLP-1 is preserved. Alogliptin is a DPP-4 inhibitor that slows the inactivation of the incretin hormones, thereby increasing their bloodstream concentrations and reducing fasting and postprandial glucose concentrations in a glucose-dependent manner in patients with type 2 diabetes mellitus. Alogliptin selectively binds to and inhibits DPP-4 but not DPP-8 or DPP-9 activity in vitro at concentrations approximating therapeutic exposures.
12.2 Pharmacodynamics
Single-dose administration of alogliptin tablets to healthy subjects resulted in a peak inhibition of DPP-4 within two to three hours after dosing. The peak inhibition of DPP-4 exceeded 93% across doses of 12.5 mg to 800 mg. Inhibition of DPP-4 remained above 80% at 24 hours for doses greater than or equal to 25 mg. Peak and total exposure over 24 hours to active GLP-1 were three- to four-fold greater with alogliptin tablets (at doses of 25 to 200 mg) than placebo. In a 16 week, double-blind, placebo-controlled study, alogliptin tablets 25 mg demonstrated decreases in postprandial glucagon while increasing postprandial active GLP-1 levels compared to placebo over an eight hour period following a standardized meal. It is unclear how these findings relate to changes in overall glycemic control in patients with type 2 diabetes mellitus. In this study, alogliptin tablets 25 mg demonstrated decreases in two hour postprandial glucose compared to placebo (-30 mg/dL versus 17 mg/dL, respectively).
Multiple-dose administration of alogliptin to patients with type 2 diabetes also resulted in a peak inhibition of DPP-4 within one to two hours and exceeded 93% across all doses (25 mg, 100 mg and 400 mg) after a single dose and after 14 days of once-daily dosing. At these doses of alogliptin tablets, inhibition of DPP-4 remained above 81% at 24 hours after 14 days of dosing.
Cardiac Electrophysiology
In a randomized, placebo-controlled, four-arm, parallel-group study, 257 subjects were administered either alogliptin 50 mg, alogliptin 400 mg, moxifloxacin 400 mg or placebo once daily for a total of seven days. No increase in corrected QT (QTc) was observed with either dose of alogliptin. At the 400 mg dose, peak alogliptin plasma concentrations were 19-fold higher than the peak concentrations following the maximum recommended clinical dose of 25 mg.
12.3 Pharmacokinetics
The pharmacokinetics of alogliptin tablets has been studied in healthy subjects and in patients with type 2 diabetes. After administration of single, oral doses up to 800 mg in healthy subjects, the peak plasma alogliptin concentration (median Tmax) occurred one to two hours after dosing. At the maximum recommended clinical dose of 25 mg, alogliptin tablets were eliminated with a mean terminal half-life (T1/2) of approximately 21 hours.
After multiple-dose administration up to 400 mg for 14 days in patients with type 2 diabetes, accumulation of alogliptin was minimal with an increase in total [e.g., area under the plasma concentration curve (AUC)] and peak (i.e., Cmax) alogliptin exposures of 34% and 9%, respectively. Total and peak exposure to alogliptin increased proportionally across single doses and multiple doses of alogliptin ranging from 25 mg to 400 mg. The intersubject coefficient of variation for alogliptin AUC was 17%. The pharmacokinetics of alogliptin tablets were also shown to be similar in healthy subjects and in patients with type 2 diabetes.
Absorption
The absolute bioavailability of alogliptin tablets is approximately 100%. Administration of alogliptin tablets with a high-fat meal results in no significant change in total and peak exposure to alogliptin. Alogliptin tablets may therefore be administered with or without food.
Distribution
Following a single, 12.5 mg intravenous infusion of alogliptin to healthy subjects, the volume of distribution during the terminal phase was 417 L, indicating that the drug is well distributed into tissues.
Alogliptin is 20% bound to plasma proteins.
Metabolism
Alogliptin does not undergo extensive metabolism and 60% to 71% of the dose is excreted as unchanged drug in the urine.
Two minor metabolites were detected following administration of an oral dose of [14C] alogliptin, N-demethylated, M-I (less than 1% of the parent compound), and N-acetylated alogliptin, M-II (less than 6% of the parent compound). M-I is an active metabolite and is an inhibitor of DPP-4 similar to the parent molecule; M-II does not display any inhibitory activity toward DPP-4 or other DPP-related enzymes. In vitro data indicate that CYP2D6 and CYP3A4 contribute to the limited metabolism of alogliptin.
Alogliptin exists predominantly as the (R)-enantiomer (more than 99%) and undergoes little or no chiral conversion in vivo to the (S)-enantiomer. The (S)-enantiomer is not detectable at the 25 mg dose.
Excretion
The primary route of elimination of [14C] alogliptin-derived radioactivity occurs via renal excretion (76%) with 13% recovered in the feces, achieving a total recovery of 89% of the administered radioactive dose. The renal clearance of alogliptin (9.6 L/hr) indicates some active renal tubular secretion and systemic clearance was 14.0 L/hr.
Special Populations
Renal Impairment
A single-dose, open-label study was conducted to evaluate the pharmacokinetics of alogliptin 50 mg in patients with chronic renal impairment compared with healthy subjects.
In patients with mild renal impairment (creatinine clearance [CrCl] ≥60 to <90 mL/min), an approximate 1.2-fold increase in plasma AUC of alogliptin was observed. Because increases of this magnitude are not considered clinically relevant, dose adjustment for patients with mild renal impairment is not recommended.
In patients with moderate renal impairment (CrCl ≥30 to <60 mL/min), an approximate two-fold increase in plasma AUC of alogliptin was observed. To maintain similar systemic exposures of alogliptin tablets to those with normal renal function, the recommended dose is 12.5 mg once daily in patients with moderate renal impairment.
In patients with severe renal impairment (CrCl ≥15 to <30 mL/min) and end-stage renal disease (ESRD) (CrCl <15 mL/min or requiring dialysis), an approximate three- and four-fold increase in plasma AUC of alogliptin were observed, respectively. Dialysis removed approximately 7% of the drug during a three-hour dialysis session. Alogliptin tablets may be administered without regard to the timing of the dialysis. To maintain similar systemic exposures of alogliptin tablets to those with normal renal function, the recommended dose is 6.25 mg once daily in patients with severe renal impairment, as well as in patients with ESRD requiring dialysis.
Hepatic Impairment
Total exposure to alogliptin was approximately 10% lower and peak exposure was approximately 8% lower in patients with moderate hepatic impairment (Child-Pugh Grade B) compared to healthy subjects. The magnitude of these reductions is not considered to be clinically meaningful. Patients with severe hepatic impairment (Child-Pugh Grade C) have not been studied. Use caution when administering alogliptin tablets to patients with liver disease [see Use in Specific Populations (8.6) and Warnings and Precautions (5.4)].
Gender
No dose adjustment of alogliptin tablets is necessary based on gender. Gender did not have any clinically meaningful effect on the pharmacokinetics of alogliptin.
Geriatric
No dose adjustment of alogliptin tablets is necessary based on age. Age did not have any clinically meaningful effect on the pharmacokinetics of alogliptin.
Drug Interactions
In Vitro Assessment of Drug Interactions
In vitro studies indicate that alogliptin is neither an inducer of CYP1A2, CYP2B6, CYP2C9, CYP2C19 and CYP3A4, nor an inhibitor of CYP1A2, CYP2C8, CYP2C9, CYP2C19, CYP3A4 and CYP2D6 at clinically relevant concentrations.
In Vivo Assessment of Drug Interactions
Effects of Alogliptin on the Pharmacokinetics of Other Drugs
In clinical studies, alogliptin did not meaningfully increase the systemic exposure to the following drugs that are metabolized by CYP isozymes or excreted unchanged in urine (Figure 1). No dose adjustment of alogliptin tablets is recommended based on results of the described pharmacokinetic studies.
Figure 1. Effect of Alogliptin on the Pharmacokinetic Exposure to Other Drugs
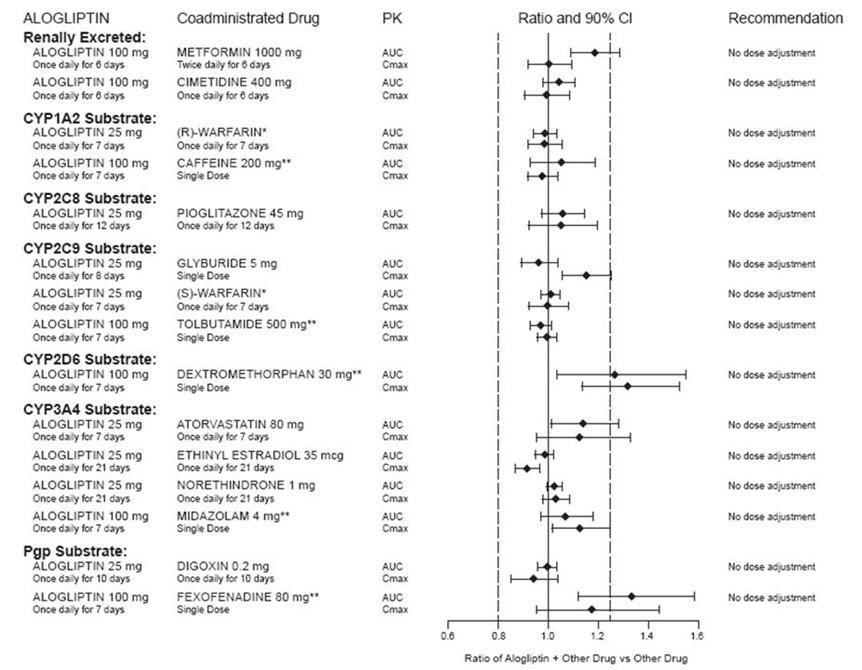
*Warfarin was given once daily at a stable dose in the range of 1 mg to 10 mg. Alogliptin had no significant effect on the prothrombin time (PT) or International Normalized Ratio (INR).
**Caffeine (1A2 substrate), tolbutamide (2C9 substrate), dextromethorphan (2D6 substrate), midazolam (3A4 substrate) and fexofenadine (P-gp substrate) were administered as a cocktail.
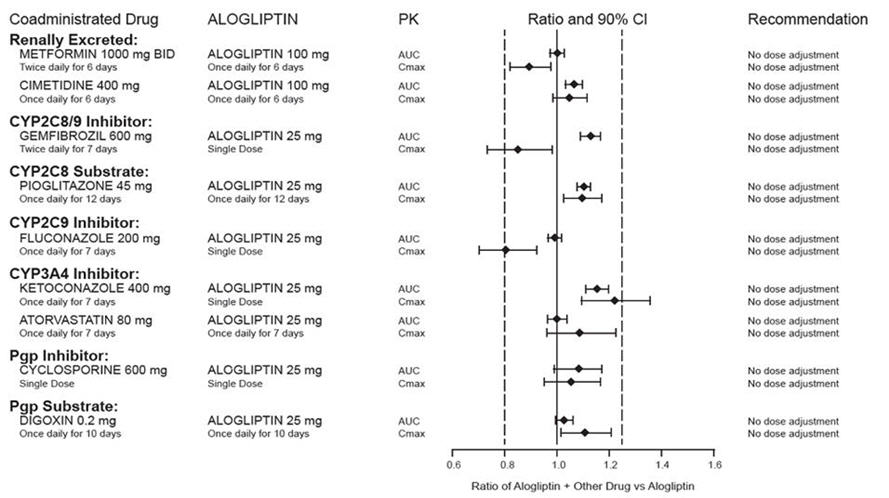
Effects of Other Drugs on the Pharmacokinetics of Alogliptin
There are no clinically meaningful changes in the pharmacokinetics of alogliptin when alogliptin tablets are administered concomitantly with the drugs described below (Figure 2).
Figure 2. Effect of Other Drugs on the Pharmacokinetic Exposure of Alogliptin
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Rats were administered oral doses of 75, 400 and 800 mg/kg alogliptin for two years. No drug-related tumors were observed up to 75 mg/kg or approximately 32 times the maximum recommended clinical dose of 25 mg, based on area under the plasma concentration curve (AUC) exposure. At higher doses (approximately 308 times the maximum recommended clinical dose of 25 mg), a combination of thyroid C-cell adenomas and carcinomas increased in male but not female rats. No drug-related tumors were observed in mice after administration of 50, 150 or 300 mg/kg alogliptin for two years, or up to approximately 51 times the maximum recommended clinical dose of 25 mg, based on AUC exposure.
Alogliptin was not mutagenic or clastogenic, with and without metabolic activation, in the Ames test with S. typhimurium and E. coli or the cytogenetic assay in mouse lymphoma cells. Alogliptin was negative in the in vivo mouse micronucleus study.
In a fertility study in rats, alogliptin had no adverse effects on early embryonic development, mating or fertility at doses up to 500 mg/kg, or approximately 172 times the clinical dose based on plasma drug exposure (AUC).
14 CLINICAL STUDIES
Alogliptin tablets have been studied as monotherapy and in combination with metformin, a sulfonylurea, a thiazolidinedione (either alone or in combination with metformin or a sulfonylurea) and insulin (either alone or in combination with metformin).
A total of 14,053 patients with type 2 diabetes were randomized in 11 double-blind, placebo- or active-controlled clinical safety and efficacy studies conducted to evaluate the effects of alogliptin tablets on glycemic control. The racial distribution of patients exposed to study medication was 70% Caucasian, 17% Asian, 6% Black and 7% other racial groups. The ethnic distribution was 30% Hispanic. Patients had an overall mean age of 57 years (range 21 to 91 years).
In patients with type 2 diabetes, treatment with alogliptin tablets produced clinically meaningful and statistically significant improvements in hemoglobin A1c (A1C) compared to placebo. As is typical for trials of agents to treat type 2 diabetes, the mean reduction in A1C with alogliptin tablets appears to be related to the degree of A1C elevation at baseline.
Alogliptin tablets had similar changes from baseline in serum lipids compared to placebo.
14.1 Patients with Inadequate Glycemic Control on Diet and Exercise
A total of 1768 patients with type 2 diabetes participated in three double-blind studies to evaluate the efficacy and safety of alogliptin tablets in patients with inadequate glycemic control on diet and exercise. All three studies had a four week, single-blind, placebo run-in period followed by a 26 week randomized treatment period. Patients who failed to meet prespecified hyperglycemic goals during the 26 week treatment periods received glycemic rescue therapy.
In a 26 week, double-blind, placebo-controlled study, a total of 329 patients (mean baseline A1C = 8%) were randomized to receive alogliptin tablets 12.5 mg, alogliptin tablets 25 mg or placebo once daily. Treatment with alogliptin tablets 25 mg resulted in statistically significant improvements from baseline in A1C and fasting plasma glucose (FPG) compared to placebo at Week 26 (Table 3). A total of 8% of patients receiving alogliptin tablets 25 mg and 30% of those receiving placebo required glycemic rescue therapy.
Improvements in A1C were not affected by gender, age or baseline body mass index (BMI).
The mean change in body weight with alogliptin tablets was similar to placebo.
| Alogliptin Tablets 25 mg | Placebo | |
|---|---|---|
| A1C (%) | N=128 | N=63 |
| Baseline (mean) | 7.9 | 8.0 |
| Change from baseline (adjusted mean†) | -0.6 | 0 |
| Difference from placebo (adjusted mean† with 95% confidence interval) | -0.6‡ (-0.8, -0.3) | ˗ |
| % of patients (n/N) achieving A1C ≤7% | 44% (58/131)‡ | 23% (15/64) |
| FPG (mg/dL) | N=129 | N=64 |
| Baseline (mean) | 172 | 173 |
| Change from baseline (adjusted mean†) | -16 | 11 |
| Difference from placebo (adjusted mean† with 95% confidence interval) | -28‡ (-40, -15) | ˗ |
In a 26-week, double-blind, active-controlled study, a total of 655 patients (mean baseline A1C = 8.8%) were randomized to receive alogliptin tablets 25 mg alone, pioglitazone 30 mg alone, alogliptin tablets 12.5 mg with pioglitazone 30 mg or alogliptin tablets 25 mg with pioglitazone 30 mg once daily. Coadministration of alogliptin tablets 25 mg with pioglitazone 30 mg resulted in statistically significant improvements from baseline in A1C and FPG compared to alogliptin tablets 25 mg alone and to pioglitazone 30 mg alone (Table 4). A total of 3% of patients receiving alogliptin tablets 25 mg coadministered with pioglitazone 30 mg, 11% of those receiving alogliptin tablets 25 mg alone and 6% of those receiving pioglitazone 30 mg alone required glycemic rescue.
Improvements in A1C were not affected by gender, age or baseline BMI.
The mean increase in body weight was similar between pioglitazone alone and alogliptin tablets when coadministered with pioglitazone.
| Alogliptin Tablets 25 mg | Pioglitazone 30 mg | Alogliptin Tablets 25 mg + Pioglitazone 30 mg |
|
|---|---|---|---|
| A1C (%) | N=160 | N=153 | N=158 |
| Baseline (mean) | 8.8 | 8.8 | 8.8 |
| Change from baseline (adjusted mean†) | -1.0 | -1.2 | -1.7 |
| Difference from alogliptin tablets 25 mg (adjusted mean† with 95% confidence interval) | ˗ | ˗ | -0.8‡ (-1.0, -0.5) |
| Difference from pioglitazone 30 mg (adjusted mean† with 95% confidence interval) | ˗ | ˗ | -0.6‡ (-0.8, -0.3) |
| % of Patients (n/N) achieving A1C ≤7% | 24% (40/164) | 34% (55/163) | 63% (103/164)‡ |
| FPG (mg/dL) | N=162 | N=157 | N=162 |
| Baseline (mean) | 189 | 189 | 185 |
| Change from baseline (adjusted mean†) | -26 | -37 | -50 |
| Difference from alogliptin tablets 25 mg (adjusted mean† with 95% confidence interval) | ˗ | ˗ | -24‡ (-34, -15) |
| Difference from pioglitazone 30 mg (adjusted mean† with 95% confidence interval) | ˗ | ˗ | -13‡ (-22, -4) |
In a 26 week, double-blind, placebo-controlled study, a total of 784 patients inadequately controlled on diet and exercise alone (mean baseline A1C = 8.4%) were randomized to one of seven treatment groups: placebo; metformin HCl 500 mg or metformin HCl 1000 mg twice daily; alogliptin tablets 12.5 mg twice daily; alogliptin tablets 25 mg daily; or alogliptin tablets 12.5 mg in combination with metformin HCl 500 mg or metformin HCl 1000 mg twice daily. Both coadministration treatment arms (alogliptin tablets 12.5 mg + metformin HCl 500 mg and alogliptin tablets 12.5 mg + metformin HCl 1000 mg) resulted in statistically significant improvements in A1C and FPG when compared with their respective individual alogliptin and metformin component regimens (Table 5). Coadministration treatment arms demonstrated improvements in two hour postprandial glucose (PPG) compared to alogliptin tablets alone or metformin alone (Table 5). A total of 12.3% of patients receiving alogliptin tablets 12.5 mg + metformin HCl 500 mg, 2.6% of patients receiving alogliptin tablets 12.5 mg + metformin HCl 1000 mg, 17.3% of patients receiving alogliptin tablets 12.5 mg, 22.9% of patients receiving metformin HCl 500 mg, 10.8% of patients receiving metformin HCl 1000 mg and 38.7% of patients receiving placebo required glycemic rescue.
Improvements in A1C were not affected by gender, age, race or baseline BMI. The mean decrease in body weight was similar between metformin alone and alogliptin tablets when coadministered with metformin.
| Placebo | Alogliptin Tablets 12.5 mg Twice Daily | Metformin HCl 500 mg Twice Daily | Metformin HCl 1000 mg Twice Daily | Alogliptin Tablets 12.5 mg + Metformin HCl 500 mg Twice Daily | Alogliptin Tablets 12.5 mg + Metformin HCl 1000 mg Twice Daily |
|
|---|---|---|---|---|---|---|
|
||||||
| A1C (%)* | N=102 | N=104 | N=103 | N=108 | N=102 | N=111 |
| Baseline (mean) | 8.5 | 8.4 | 8.5 | 8.4 | 8.5 | 8.4 |
| Change from baseline (adjusted mean†) | 0.1 | -0.6 | -0.7 | -1.1 | -1.2 | -1.6 |
| Difference from metformin (adjusted mean† with 95% confidence interval) | - | - | - | - | -0.6‡
(-0.9, -0.3) | -0.4‡
(-0.7, -0.2) |
| Difference from Alogliptin Tablets (adjusted mean† with 95% confidence interval) | - | - | - | - | -0.7‡
(-1.0, -0.4) | -1.0‡
(-1.3, -0.7) |
| % of Patients (n/N) achieving A1C <7%§ | 4% (4/102) | 20% (21/104) | 27% (28/103) | 34% (37/108) | 47%‡
(48/102) | 59%‡
(66/111) |
| FPG (mg/dL)* | N=105 | N=106 | N=106 | N=110 | N=106 | N=112 |
| Baseline (mean) | 187 | 177 | 180 | 181 | 176 | 185 |
| Change from baseline (adjusted mean†) | 12 | -10 | -12 | -32 | -32 | -46 |
| Difference from metformin (adjusted mean† with 95% confidence interval) | - | - | - | - | -20‡
(-33, -8) | -14‡
(-26, -2) |
| Difference from Alogliptin Tablets (adjusted mean† with 95% confidence interval) | - | - | - | - | -22‡
(-35, -10) | -36‡
(-49, -24) |
| 2-Hour PPG (mg/dL)¶ | N=26 | N=34 | N=28 | N=37 | N=31 | N=37 |
| Baseline (mean) | 263 | 272 | 247 | 266 | 261 | 268 |
| Change from baseline (adjusted mean†) | -21 | -43 | -49 | -54 | -68 | -86‡ |
| Difference from metformin (adjusted mean† with 95% confidence interval) | - | - | - | - | -19 (-49, 11) | -32‡
(-58, -5) |
| Difference from Alogliptin Tablets (adjusted mean† with 95% confidence interval) | - | - | - | - | -25 (-53, -3) | -43‡
(-70, -16) |
14.2 Combination Therapy
Add-On Therapy to Metformin
A total of 2081 patients with type 2 diabetes participated in two 26 week, double-blind, placebo-controlled studies to evaluate the efficacy and safety of alogliptin tablets as add-on therapy to metformin. In both studies, patients were inadequately controlled on metformin at a dose of at least 1500 mg per day or at the maximum tolerated dose. All patients entered a four week, single-blind placebo run-in period prior to randomization. Patients who failed to meet prespecified hyperglycemic goals during the 26 week treatment periods received glycemic rescue therapy.
In the first 26 week, placebo-controlled study, a total of 527 patients already on metformin (mean baseline A1C = 8%) were randomized to receive alogliptin tablets 12.5 mg, alogliptin tablets 25 mg or placebo. Patients were maintained on a stable dose of metformin (median dose = 1700 mg) during the treatment period. Alogliptin tablets 25 mg in combination with metformin resulted in statistically significant improvements from baseline in A1C and FPG at Week 26, when compared to placebo (Table 6). A total of 8% of patients receiving alogliptin tablets 25 mg and 24% of patients receiving placebo required glycemic rescue.
Improvements in A1C were not affected by gender, age, baseline BMI or baseline metformin dose.
The mean decrease in body weight was similar between alogliptin tablets and placebo when given in combination with metformin.
| Alogliptin Tablets 25 mg + Metformin | Placebo + Metformin | |
|---|---|---|
| A1C (%) | N=203 | N=103 |
| Baseline (mean) | 7.9 | 8.0 |
| Change from baseline (adjusted mean†) | -0.6 | -0.1 |
| Difference from placebo (adjusted mean† with 95% confidence interval) | -0.5‡ (-0.7, -0.3) | ˗ |
| % of patients (n/N) achieving A1C ≤7% | 44% (92/207)‡ | 18% (19/104) |
| FPG (mg/dL) | N=204 | N=104 |
| Baseline (mean) | 172 | 180 |
| Change from baseline (adjusted mean†) | -17 | 0 |
| Difference from placebo (adjusted mean† with 95% confidence interval) | -17‡ (-26, -9) | ˗ |
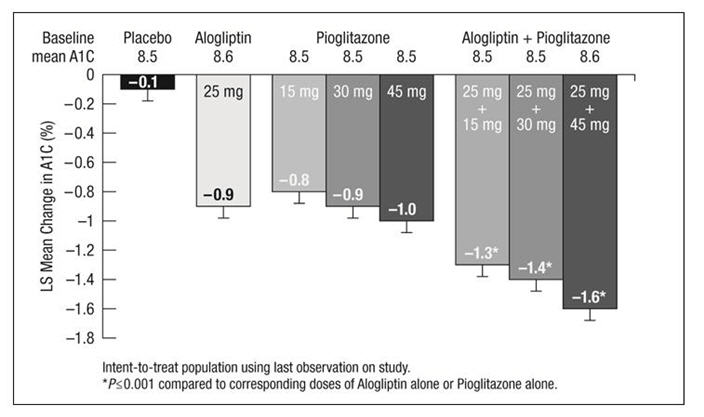
In the second 26 week, double-blind, placebo-controlled study, a total of 1554 patients already on metformin (mean baseline A1C = 8.5%) were randomized to one of 12 double-blind treatment groups: placebo; 12.5 mg or 25 mg of alogliptin tablets alone; 15 mg, 30 mg or 45 mg of pioglitazone alone; or 12.5 mg or 25 mg of alogliptin tablets in combination with 15 mg, 30 mg or 45 mg of pioglitazone. Patients were maintained on a stable dose of metformin (median dose = 1700 mg) during the treatment period. Coadministration of alogliptin tablets and pioglitazone provided statistically significant improvements in A1C and FPG compared to placebo, to alogliptin tablets alone or to pioglitazone alone when added to background metformin therapy (Table 7, Figure 3). In addition, improvements from baseline A1C were comparable between alogliptin tablets alone and pioglitazone alone (15 mg, 30 mg and 45 mg) at Week 26. A total of 4%, 5% or 2% of patients receiving alogliptin tablets 25 mg with 15 mg, 30 mg or 45 mg pioglitazone, 33% of patients receiving placebo, 13% of patients receiving alogliptin tablets 25 mg and 10%, 15% or 9% of patients receiving pioglitazone 15 mg, 30 mg or 45 mg alone required glycemic rescue.
Improvements in A1C were not affected by gender, age or baseline BMI.
The mean increase in body weight was similar between pioglitazone alone and alogliptin tablets when coadministered with pioglitazone.
| Placebo | Alogliptin Tablets 25 mg | Pioglitazone 15 mg | Pioglitazone 30 mg | Pioglitazone 45 mg | Alogliptin Tablets 25 mg + Pioglitazone 15 mg | Alogliptin Tablets 25 mg + Pioglitazone 30 mg | Alogliptin Tablets 25 mg + Pioglitazone 45 mg | |
|---|---|---|---|---|---|---|---|---|
| A1C (%) | N=126 | N=123 | N=127 | N=123 | N=126 | N=127 | N=124 | N=126 |
| Baseline (mean) | 8.5 | 8.6 | 8.5 | 8.5 | 8.5 | 8.5 | 8.5 | 8.6 |
| Change from baseline (adjusted mean†) | -0.1 | -0.9 | -0.8 | -0.9 | -1.0 | -1.3‡ | -1.4‡ | -1.6‡ |
| Difference from pioglitazone (adjusted mean† with 95% confidence interval) | - | - | - | - | -0.5‡
(-0.7, -0.3) | -0.5‡
(-0.7, -0.3) | -0.6‡
(-0.8, -0.4) |
|
| Difference from Alogliptin Tablets (adjusted mean† with 95% confidence interval) | - | - | - | - | - | -0.4‡
(-0.6, -0.1) | -0.5‡
(-0.7, -0.3) | -0.7‡
(-0.9, -0.5) |
| Patients (%) achieving A1C ≤7% | 6% (8/129) | 27% (35/129) | 26% (33/129) | 30% (38/129) | 36% (47/129) | 55% (71/130)‡ | 53% (69/130)‡ | 60% (78/130)‡ |
| FPG (mg/dL) | N=129 | N=126 | N=127 | N=125 | N=129 | N=130 | N=126 | N=127 |
| Baseline (mean) | 177 | 184 | 177 | 175 | 181 | 179 | 179 | 178 |
| Change from baseline (adjusted mean†) | 7 | -19 | -24 | -29 | -32 | -38‡ | -42‡ | -53‡ |
| Difference from pioglitazone (adjusted mean† with 95% confidence interval) | - | - | - | - | - | -14‡ (-24, -5) | -13‡ (-23, -3) | -20‡ (-30, -11) |
| Difference from Alogliptin Tablets (adjusted mean† with 95% confidence interval) | - | - | - | - | - | -19‡ (-29, -10) | -23‡ (-33, -13) | -34‡ (-44, -24) |
Figure 3. Change from Baseline in A1C at Week 26 with Alogliptin Tablets and Pioglitazone Alone and Alogliptin Tablets in Combination with Pioglitazone When Added to Metformin
Add-On Therapy to a Thiazolidinedione
In a 26 week, placebo-controlled study, a total of 493 patients inadequately controlled on a thiazolidinedione alone or in combination with metformin or a sulfonylurea (10 mg) (mean baseline A1C = 8%) were randomized to receive alogliptin tablets 12.5 mg, alogliptin tablets 25 mg or placebo. Patients were maintained on a stable dose of pioglitazone (median dose = 30 mg) during the treatment period; those who were also previously treated on metformin (median dose = 2000 mg) or sulfonylurea (median dose = 10 mg) prior to randomization were maintained on the combination therapy during the treatment period. All patients entered into a four week, single-blind placebo run-in period prior to randomization. Patients who failed to meet prespecified hyperglycemic goals during the 26 week treatment period received glycemic rescue therapy.
The addition of alogliptin tablets 25 mg once daily to pioglitazone therapy resulted in statistically significant improvements from baseline in A1C and FPG at Week 26, compared to placebo (Table 8). A total of 9% of patients who were receiving alogliptin tablets 25 mg and 12% of patients receiving placebo required glycemic rescue.
Improvements in A1C were not affected by gender, age, baseline BMI or baseline pioglitazone dose.
Clinically meaningful reductions in A1C were observed with alogliptin tablets compared to placebo regardless of whether subjects were receiving concomitant metformin or sulfonylurea (-0.2% placebo versus -0.9% alogliptin tablets) therapy or pioglitazone alone (0% placebo versus -0.52% alogliptin tablets).
The mean increase in body weight was similar between alogliptin tablets and placebo when given in combination with pioglitazone.
| Alogliptin Tablets 25 mg + Pioglitazone ± Metformin ± Sulfonylurea | Placebo + Pioglitazone ± Metformin ± Sulfonylurea | |
|---|---|---|
|
||
| A1C (%) | N=195 | N=95 |
| Baseline (mean) | 8 | 8 |
| Change from baseline (adjusted mean†) | -0.8 | -0.2 |
| Difference from placebo (adjusted mean† with 95% confidence interval) | -0.6‡ (-0.8, -0.4) | ˗ |
| % of patients (n/N) achieving A1C ≤7% | 49% (98/199)‡ | 34% (33/97) |
| FPG (mg/dL) | N=197 | N=97 |
| Baseline (mean) | 170 | 172 |
| Change from baseline (adjusted mean†) | -20 | -6 |
| Difference from placebo (adjusted mean† with 95% confidence interval) | -14‡ (-23, -5) | ˗ |
Add-on Combination Therapy with Pioglitazone and Metformin
In a 52 week, active-comparator study, a total of 803 patients inadequately controlled (mean baseline A1C = 8.2%) on a current regimen of pioglitazone 30 mg and metformin at least 1500 mg per day or at the maximum tolerated dose were randomized to either receive the addition of alogliptin tablets 25 mg or the titration of pioglitazone 30 mg to 45 mg following a four week, single-blind placebo run-in period. Patients were maintained on a stable dose of metformin (median dose = 1700 mg). Patients who failed to meet prespecified hyperglycemic goals during the 52 week treatment period received glycemic rescue therapy.
In combination with pioglitazone and metformin, alogliptin tablets 25 mg were shown to be statistically superior in lowering A1C and FPG compared with the titration of pioglitazone from 30 mg to 45 mg at Week 26 and at Week 52 (Table 9; results shown only for Week 52). A total of 11% of patients in the alogliptin tablets 25 mg treatment group and 22% of patients in the pioglitazone up-titration group required glycemic rescue.
Improvements in A1C were not affected by gender, age, race or baseline BMI.
The mean increase in body weight was similar in both treatment arms.
| Alogliptin Tablets 25 mg + Pioglitazone 30 mg + Metformin | Pioglitazone 45 mg + Metformin | |
|---|---|---|
|
||
| A1C (%) | N=397 | N=394 |
| Baseline (mean) | 8.2 | 8.1 |
| Change from baseline (adjusted mean†) | -0.7 | -0.3 |
| Difference from pioglitazone 45 mg + metformin (adjusted mean† with 95% confidence interval) | -0.4‡ (-0.5, -0.3) | ˗ |
| % of Patients (n/N) achieving A1C≤7% | 33% (134/404)§ | 21% (85/399) |
| Fasting Plasma Glucose (mg/dL)‡ | N=399 | N=396 |
| Baseline (mean) | 162 | 162 |
| Change from baseline (adjusted mean†) | -15 | -4 |
| Difference from pioglitazone 45 mg + metformin (adjusted mean† with 95% confidence interval) | -11§ (-16, -6) | ˗ |
Add-On Therapy to a Sulfonylurea
In a 26 week, placebo-controlled study, a total of 500 patients inadequately controlled on a sulfonylurea (mean baseline A1C = 8.1%) were randomized to receive alogliptin tablets 12.5 mg, alogliptin tablets 25 mg or placebo. Patients were maintained on a stable dose of glyburide (median dose = 10 mg) during the treatment period. All patients entered into a four week, single-blind, placebo run-in period prior to randomization. Patients who failed to meet prespecified hyperglycemic goals during the 26 week treatment period received glycemic rescue therapy.
The addition of alogliptin tablets 25 mg to glyburide therapy resulted in statistically significant improvements from baseline in A1C at Week 26 when compared to placebo (Table 10). Improvements in FPG observed with alogliptin tablets 25 mg were not statistically significant compared with placebo. A total of 16% of patients receiving alogliptin tablets 25 mg and 28% of those receiving placebo required glycemic rescue.
Improvements in A1C were not affected by gender, age, baseline BMI or baseline glyburide dose.
The mean change in body weight was similar between alogliptin tablets and placebo when given in combination with glyburide.
| Alogliptin Tablets 25 mg + Glyburide | Placebo + Glyburide | |
|---|---|---|
| A1C (%) | N=197 | N=97 |
| Baseline (mean) | 8.1 | 8.2 |
| Change from baseline (adjusted mean†) | -0.5 | 0 |
| Difference from placebo (adjusted mean† with 95% confidence interval) | -0.5‡ (-0.7, -0.3) | ˗ |
| % of Patients (n/N) achieving A1C ≤7% | 35% (69/198)‡ | 18% (18/99) |
| FPG (mg/dL) | N=198 | N=99 |
| Baseline (mean) | 174 | 177 |
| Change from baseline (adjusted mean†) | -8 | 2 |
| Difference from placebo (adjusted mean† with 95% confidence interval) | -11 (-22, 1) | ˗ |
Add-On Therapy to Insulin
In a 26 week, placebo-controlled study, a total of 390 patients inadequately controlled on insulin alone (42%) or in combination with metformin (58%) (mean baseline A1C = 9.3%) were randomized to receive alogliptin tablets 12.5 mg, alogliptin tablets 25 mg or placebo. Patients were maintained on their insulin regimen (median dose = 55 IU) upon randomization and those previously treated with insulin in combination with metformin (median dose = 1700 mg) prior to randomization continued on the combination regimen during the treatment period. Patients entered the trial on short-, intermediate- or long-acting (basal) insulin or premixed insulin. Patients who failed to meet prespecified hyperglycemic goals during the 26 week treatment period received glycemic rescue therapy.
The addition of alogliptin tablets 25 mg once daily to insulin therapy resulted in statistically significant improvements from baseline in A1C and FPG at Week 26, when compared to placebo (Table 11). A total of 20% of patients receiving alogliptin tablets 25 mg and 40% of those receiving placebo required glycemic rescue.
Improvements in A1C were not affected by gender, age, baseline BMI or baseline insulin dose. Clinically meaningful reductions in A1C were observed with alogliptin tablets compared to placebo regardless of whether subjects were receiving concomitant metformin and insulin (-0.2% placebo versus -0.8% alogliptin tablets) therapy or insulin alone (0.1% placebo versus -0.7% alogliptin tablets).
The mean increase in body weight was similar between alogliptin tablets and placebo when given in combination with insulin.
| Alogliptin Tablets 25 mg + Insulin ± Metformin | Placebo + Insulin ± Metformin | |
|---|---|---|
| A1C (%) | N=126 | N=126 |
| Baseline (mean) | 9.3 | 9.3 |
| Change from baseline (adjusted mean†) | -0.7 | -0.1 |
| Difference from placebo (adjusted mean† with 95% confidence interval) | -0.6‡ (-0.8, -0.4) | ˗ |
| % of patients (n/N) achieving A1C ≤7% | 8% (10/129) | 1% (1/129) |
| FPG (mg/dL) | N=128 | N=127 |
| Baseline (mean) | 186 | 196 |
| Change from baseline (adjusted mean†) | -12 | 6 |
| Difference from placebo (adjusted mean† with 95% confidence interval) | -18‡ (-33, -2) | ˗ |
14.3 Cardiovascular Safety Trial
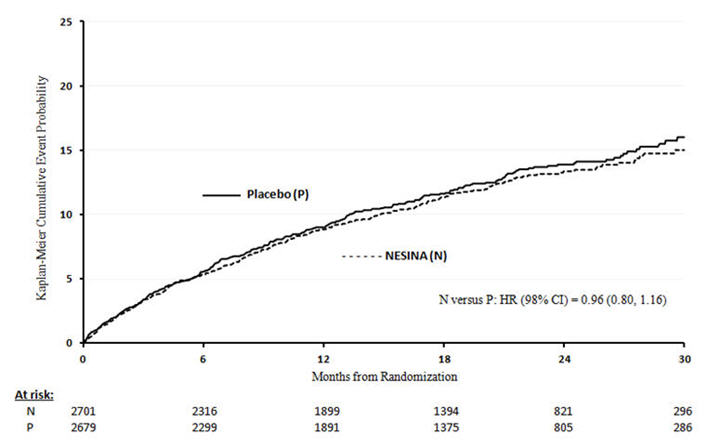
A randomized, double-blind, placebo-controlled cardiovascular outcomes trial (EXAMINE) was conducted to evaluate the cardiovascular risk of alogliptin tablets. The trial compared the risk of major adverse cardiovascular events (MACE) between alogliptin tablets (N=2701) and placebo (N=2679) when added to standard of care therapies for diabetes and atherosclerotic vascular disease (ASCVD). The trial was event driven and patients were followed until a sufficient number of primary outcome events accrued.
Eligible patients were adults with type 2 diabetes who had inadequate glycemic control at baseline (e.g., HbA1c >6.5%) and had been hospitalized for an acute coronary syndrome event (e.g., acute myocardial infarction or unstable angina requiring hospitalization) 15 to 90 days prior to randomization. The dose of alogliptin tablets was based on estimated renal function at baseline per dosage and administration recommendations [see Dosage and Administration (2.2)]. The average time between an acute coronary syndrome event and randomization was approximately 48 days.
The mean age of the population was 61 years. Most patients were male (68%), Caucasian (73%), and were recruited from outside of the United States (86%). Asian and Black patients contributed 20% and 4% of the total population, respectively. At the time of randomization patients had a diagnosis of type 2 diabetes mellitus for approximately 9 years, 87% had a prior myocardial infarction and 14% were current smokers. Hypertension (83%) and renal impairment (27% with an eGFR ≤60 ml/min/1.73 m2) were prevalent co-morbid conditions. Use of medications to treat diabetes (e.g., metformin 73%, sulfonylurea 54%, insulin 41%), and ASCVD (e.g., statin 94%, aspirin 93%, renin-angiotensin system blocker 88%, beta-blocker 87%) was similar between patients randomized to alogliptin tablets and placebo at baseline. During the trial, medications to treat diabetes and ASCVD could be adjusted to ensure care for these conditions adhered to standard of care recommendations set by local practice guidelines.
The primary endpoint in EXAMINE was the time to first occurrence of a MACE defined as the composite of cardiovascular death, nonfatal myocardial infarction (MI), or nonfatal stroke. The study was designed to exclude a pre-specified risk margin of 1.3 for the hazard ratio of MACE. The median exposure to study drug was 526 days and 95% of the patients were followed to study completion or death.
Table 12 shows the study results for the primary MACE composite endpoint and the contribution of each component to the primary MACE endpoint. The upper bound of the confidence interval was 1.16 and excluded a risk margin larger than 1.3.
|
|||||
| Composite of first event of CV death, nonfatal MI or nonfatal stroke (MACE) | Alogliptin | Placebo | Hazard Ratio | ||
| Number of Patients (%) | Rate per 100 PY* | Number of Patients (%) | Rate per 100 PY* | (98% CI) | |
| N=2701 | N=2679 | ||||
| 305 (11.3) | 7.6 | 316 (11.8) | 7.9 | 0.96 (0.80, 1.16) | |
| CV Death | 89 (3.3) | 2.2 | 111 (4.1) | 2.8 | |
| Non-fatal MI | 187 (6.9) | 4.6 | 173 (6.5) | 4.3 | |
| Non-fatal stroke | 29 (1.1) | 0.7 | 32 (1.2) | 0.8 | |
The Kaplan-Meier based cumulative event probability is presented in Figure 4 for the time to first occurrence of the primary MACE composite endpoint by treatment arm. The curves for placebo and alogliptin tablets overlap throughout the duration of the study. The observed incidence of MACE was highest within the first 60 days after randomization in both treatment arms (14.8 MACE per 100 PY), decreased from day 60 to the end of the first year (8.4 per 100 PY) and was lowest after one year of follow-up (5.2 per 100 PY).
| Figure 4. Observed Cumulative Rate of MACE in EXAMINE |
 |
The rate of all cause death was similar between treatment arms with 153 (3.6 per 100 PY) recorded among patients randomized to alogliptin tablets and 173 (4.1 per 100 PY) among patients randomized to placebo. A total of 112 deaths (2.9 per 100 PY) among patients on alogliptin tablets and 130 among patients on placebo (3.5 per 100 PY) were adjudicated as cardiovascular deaths.
16 HOW SUPPLIED/STORAGE AND HANDLING
Product: 50090-5574
NDC: 50090-5574-0 30 TABLET, FILM COATED in a BOTTLE
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Pancreatitis
Inform patients that acute pancreatitis has been reported during use of alogliptin tablets. Educate patients that persistent, severe abdominal pain, sometimes radiating to the back, which may or may not be accompanied by vomiting, is the hallmark symptom of acute pancreatitis. Instruct patients to promptly discontinue alogliptin tablets and contact their physician if persistent severe abdominal pain occurs.
Heart Failure
Inform patients of the signs and symptoms of heart failure. Before initiating alogliptin tablets, patients should be asked about a history of heart failure or other risk factors for heart failure including moderate to severe renal impairment. Instruct patients to contact their healthcare providers as soon as possible if they experience symptoms of heart failure, including increasing shortness of breath, rapid increase in weight, or swelling of the feet.
Hypersensitivity Reactions
Inform patients that allergic reactions have been reported during use of alogliptin tablets. Instruct patients if symptoms of allergic reactions (including skin rash, hives and swelling of the face, lips, tongue and throat that may cause difficulty in breathing or swallowing) occur, patients should discontinue alogliptin tablets and seek medical advice promptly.
Hepatic Effects
Inform patients that postmarketing reports of liver injury, sometimes fatal, have been reported during use of alogliptin tablets. Instruct patients if signs or symptoms of liver injury occur, patients should discontinue alogliptin tablets and seek medical advice promptly.
Hypoglycemia with Concomitant Use with Insulin or Insulin Secretagogues
Inform patients that hypoglycemia can occur, particularly when an insulin secretagogue or insulin is used in combination with alogliptin tablets. Educate patients about the risks, symptoms and appropriate management of hypoglycemia.
Severe and Disabling Arthralgia
Inform patients that severe and disabling joint pain may occur with this class of drugs. The time to onset of symptoms can range from one day to years. Instruct patients to seek medical advice if severe joint pain occurs.
Bullous Pemphigoid
Inform patients that bullous pemphigoid may occur with this class of drugs. Instruct patients to seek medical advice if blisters or erosions occur [see Warnings and Precautions (5.7)].
| ALO332 R6 | |||||
| This Medication Guide has been approved by the U.S. Food and Drug Administration. | 03/2022 | ||||
| MEDICATION GUIDE Alogliptin Tablets |
|||||
| Read this Medication Guide carefully before you start taking alogliptin tablets and each time you get a refill. There may be new information. This information does not take the place of talking with your doctor about your medical condition or treatment. If you have any questions about alogliptin tablets, ask your doctor or pharmacist. | |||||
| What is the most important information I should know about alogliptin tablets? Serious side effects can happen to people taking alogliptin tablets, including: |
|||||
|
|||||
|
|
|
|||
| Stop taking alogliptin tablets and call your doctor right away if you have pain in your stomach area (abdomen) that is severe and will not go away. The pain may be felt going from your abdomen through to your back. The pain may happen with or without vomiting. These may be symptoms of pancreatitis. | |||||
|
|||||
|
|
|
|||
| These may be symptoms of heart failure. | |||||
| What are alogliptin tablets? | |||||
|
|||||
| It is not known if alogliptin tablets are safe and effective in children under the age of 18. | |||||
| Who should not take alogliptin tablets? Do not take alogliptin tablets if you: |
|||||
|
|||||
|
|
||||
| If you have any of these symptoms, stop taking alogliptin tablets and contact your doctor or go to the nearest hospital emergency room right away. | |||||
| What should I tell my doctor before and during treatment with alogliptin tablets? | |||||
| Before you take alogliptin tablets, tell your doctor if you: | |||||
|
|||||
| Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements. | |||||
| Know the medicines you take. Keep a list of them and show it to your doctor and pharmacist before you start any new medicine. | |||||
| Alogliptin tablets may affect the way other medicines work, and other medicines may affect how alogliptin tablets works. Contact your doctor before you start or stop other types of medicines. | |||||
| How should I take alogliptin tablets? | |||||
|
|||||
| What are the possible side effects of alogliptin tablets? | |||||
| Alogliptin tablets can cause serious side effects, including: | |||||
| See "What is the most important information I should know about alogliptin tablets?" | |||||
|
|||||
|
|
||||
| If you have these symptoms, stop taking alogliptin tablets and contact your doctor right away or go the nearest hospital emergency room. | |||||
|
|||||
|
|
|
|||
|
|||||
|
|
|
|
|
|
|
|||||
| The most common side effects of alogliptin tablets include stuffy or runny nose and sore throat, headache, or cold-like symptoms (upper respiratory tract infection). | |||||
| Tell your doctor if you have any side effect that bothers you or that does not go away. | |||||
| These are not all the possible side effects of alogliptin tablets. For more information, ask your doctor or pharmacist. | |||||
| Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | |||||
| How should I store alogliptin tablets? | |||||
| Store alogliptin tablets at room temperature between 68°F to 77°F (20°C to 25°C). | |||||
| Keep alogliptin tablets and all medicines out of the reach of children. | |||||
| General information about the safe and effective use of alogliptin tablets. | |||||
| Medicines are sometimes prescribed for purposes other than those listed in the Medication Guide. Do not take alogliptin tablets for a condition for which it was not prescribed. Do not give alogliptin tablets to other people, even if they have the same symptoms you have. It may harm them. | |||||
| This Medication Guide summarizes the most important information about alogliptin tablets. If you would like to know more information, talk with your doctor. You can ask your doctor or pharmacist for information about alogliptin tablets that is written for health professionals. | |||||
| For more information go to www.padagis.com or call 1-877-TAKEDA-7 (1-877-825-3327). | |||||
| What are the ingredients in alogliptin tablets?
Active ingredient: alogliptin Inactive ingredients: mannitol, microcrystalline cellulose, hydroxypropyl cellulose, croscarmellose sodium and magnesium stearate. In addition, the film-coating contains the following inactive ingredients: hypromellose, titanium dioxide, ferric oxide (red or yellow) and polyethylene glycol and is marked with gray F1 printing ink |
|||||
| Distributed by: Padagis™ Allegan, MI 49010 • www.padagis.com |
|||||
| All trademarks are the property of their respective owners. | |||||
