FULL PRESCRIBING INFORMATION
WARNING: HEPATOTOXICITY and EMBRYOFETAL TOXICITY
-
Hepatotoxicity
Clinically significant and potentially life-threatening liver injury, including acute liver failure requiring transplant, has been reported in patients treated with teriflunomide in the postmarketing setting [see Warnings and Precautions (5.1)]. Concomitant use of teriflunomide with other hepatotoxic drugs may increase the risk of severe liver injury.Obtain transaminase and bilirubin levels within 6 months before initiation of teriflunomide therapy. Monitor ALT levels at least monthly for six months after starting teriflunomide [see Warnings and Precautions (5.1)]. If drug induced liver injury is suspected, discontinue teriflunomide and start an accelerated elimination procedure with cholestyramine or charcoal [see Warnings and Precautions (5.3)]. Teriflunomide is contraindicated in patients with severe hepatic impairment [see Contraindications (4)]. Patients with pre-existing liver disease may be at increased risk of developing elevated serum transaminases when taking teriflunomide.
-
Embryofetal Toxicity
Teriflunomide is contraindicated for use in pregnant women and in females of reproductive potential who are not using effective contraception because of the potential for fetal harm. Teratogenicity and embryolethality occurred in animals at plasma teriflunomide exposures lower than that in humans. Exclude pregnancy before the start of treatment with teriflunomide in females of reproductive potential. Advise females of reproductive potential to use effective contraception during teriflunomide treatment and during an accelerated drug elimination procedure after teriflunomide treatment. Stop teriflunomide and use an accelerated drug elimination procedure if the patient becomes pregnant [see Contraindications (4), Warnings and Precautions (5.2, 5.3), Use in Specific Populations (8.1, 8.3),and Clinical Pharmacology (12.3)].
1 INDICATIONS AND USAGE
Teriflunomide tablet is indicated for the treatment of relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.
2 DOSAGE AND ADMINISTRATION
The recommended dose of teriflunomide tablet is 7 mg or 14 mg orally once daily. Teriflunomide tablet can be taken with or without food. Monitoring to Assess Safety.
- Obtain transaminase and bilirubin levels within 6 months before initiation of teriflunomide tablet therapy. Monitor ALT levels at least monthly for six months after starting teriflunomide tablets [see Warnings and Precautions (5.1)].
- Obtain a complete blood cell count (CBC) within 6 months before the initiation of treatment with teriflunomide tablets. Further monitoring should be based on signs and symptoms of infection [see Warnings and Precautions (5.4)].
- Prior to initiating teriflunomide tablets, screen patients for latent tuberculosis infection with a tuberculin skin test or blood test for mycobacterium tuberculosis infection [see Warnings and Precautions (5.4)].
- Exclude pregnancy prior to initiation of treatment with teriflunomide tablets in females of reproductive potential [see Warnings and Precautions (5.2)].
- Check blood pressure before start of teriflunomide tablets treatment and periodically thereafter [see Warnings and Precautions (5.9)].
3 DOSAGE FORMS AND STRENGTHS
Teriflunomide is available as 7 mg and 14 mg tablets.
The 14 mg tablet is a white to off white, pentagonal, film coated tablet debossed with B on one side and 14 on the other side. Each tablet contains 14 mg of teriflunomide.
The 7 mg tablet is a white to off white, hexagonal, film coated tablet debossed with B on one side and 7 on the other side. Each tablet contains 7 mg of teriflunomide.
4 CONTRAINDICATIONS
Teriflunomide tablets are contraindicated in/with:
- Patients with severe hepatic impairment [see Warnings and Precautions (5.1)].
- Pregnant women and females of reproductive potential not using effective contraception. Teriflunomide tablet may cause fetal harm [see Warnings and Precautions (5.2, 5.3) and Use in Specific Populations (8.1)].
- Patients with a history of a hypersensitivity reaction to teriflunomide, leflunomide, or to any of the inactive ingredients in teriflunomide tablet. Reactions have included anaphylaxis, angioedema, and serious skin reactions [see Warnings and Precautions (5.5)].
- Coadministration with leflunomide [see Clinical Pharmacology (12.3)].
5 WARNINGS AND PRECAUTIONS
5.1 Hepatotoxicity
Clinically significant and potentially life-threatening liver injury, including acute liver failure requiring transplant, has been reported in patients treated with teriflunomide in the postmarketing setting. Patients with pre-existing liver disease and patients taking other hepatotoxic drugs may be at increased risk for developing liver injury when taking teriflunomide. Clinically significant liver injury can occur at any time during treatment with teriflunomide.
Patients with pre-existing acute or chronic liver disease, or those with serum alanine aminotransferase (ALT) greater than two times the upper limit of normal (ULN) before initiating treatment, should not normally be treated with teriflunomide. Teriflunomide is contraindicated in patients with severe hepatic impairment [see Contraindications (4)].
In placebo-controlled trials in adult patients, ALT greater than three times the ULN occurred in 61/1045 (5.8%) and 62/1002 (6.2%) of patients receiving teriflunomide 7 mg and 14 mg, respectively, and 38/997 (3.8%) of patients receiving placebo, during the treatment period. These elevations occurred mostly within the first year of treatment. Half of the cases returned to normal without drug discontinuation. In clinical trials, if ALT elevation was greater than three times the ULN on two consecutive tests, teriflunomide was discontinued and patients underwent an accelerated elimination procedure [see Warnings and Precautions (5.3)]. Of the patients who underwent discontinuation and accelerated elimination in controlled trials, half returned to normal or near normal values within 2 months.
One patient in the controlled trials in adult patients developed ALT 32 times the ULN and jaundice 5 months after initiation of teriflunomide 14 mg treatment. The patient was hospitalized for 5 weeks and recovered after plasmapheresis and cholestyramine accelerated elimination procedure. Teriflunomide-induced liver injury in this patient could not be ruled out.
Obtain serum transaminase and bilirubin levels within 6 months before initiation of teriflunomide therapy. Monitor ALT levels at least monthly for six months after starting teriflunomide. Consider additional monitoring when teriflunomide is given with other potentially hepatotoxic drugs.
Consider discontinuing teriflunomide if serum transaminase increase (greater than three times the ULN) is confirmed. Monitor serum transaminase and bilirubin on teriflunomide therapy, particularly in patients who develop symptoms suggestive of hepatic dysfunction, such as unexplained nausea, vomiting, abdominal pain, fatigue, anorexia, or jaundice and/or dark urine. If liver injury is suspected to be teriflunomide-induced, discontinue teriflunomide and start an accelerated elimination procedure [see Warnings and Precautions (5.3)] and monitor liver tests weekly until normalized. If teriflunomide-induced liver injury is unlikely because some other probable cause has been found, resumption of teriflunomide therapy may be considered.
5.2 Embryofetal Toxicity
Teriflunomide may cause fetal harm when administered to a pregnant woman. Teratogenicity and embryofetal lethality occurred in animal reproduction studies in multiple animal species at plasma teriflunomide exposures similar to or lower than that in humans at the maximum recommended human dose (MRHD) of 14 mg/day [see Use in Specific Populations (8.1)].
Teriflunomide is contraindicated for use in pregnant women and in females of reproductive potential not using effective contraception [see Contraindications (4)]. Exclude pregnancy before starting treatment with teriflunomide in females of reproductive potential [see Dosage and Administration (2) ]. Advise females of reproductive potential to use effective contraception during teriflunomide treatment and during an accelerated drug elimination procedure after teriflunomide treatment [see Use in Specific Populations (8.3)]. If a woman becomes pregnant while taking teriflunomide, stop treatment with teriflunomide, apprise the patient of the potential risk to a fetus, and perform an accelerated drug elimination procedure to achieve a plasma teriflunomide concentration of less than 0.02 mg/L [see Warnings and Precautions (5.3)].
Upon discontinuing teriflunomide, it is recommended that all females of reproductive potential undergo an accelerated drug elimination procedure. Women receiving teriflunomide treatment who wish to become pregnant must discontinue teriflunomide and undergo an accelerated drug elimination procedure, which includes verification that plasma concentrations of teriflunomide are less than 0.02 mg/L (0.02 mcg/mL). Men wishing to father a child should also discontinue use of teriflunomide and either undergo an accelerated elimination procedure or wait until verification that the plasma teriflunomide concentration is less than 0.02 mg/L (0.02 mcg/mL) [see Use in Specific Populations (8.3)]. Based on animal data, human plasma concentrations of teriflunomide of less than 0.02 mg/L (0.02 mcg/mL) are expected to have minimal embryofetal risk [see Contraindications (4), Warnings and Precautions (5.3), and Use in Specific Populations (8.1)].
5.3 Procedure for Accelerated Elimination of Teriflunomide
Teriflunomide is eliminated slowly from the plasma [see Clinical Pharmacology (12.3)]. Without an accelerated elimination procedure, it takes on average 8 months to reach plasma concentrations less than 0.02 mg/L, although because of individual variations in drug clearance it may take as long as 2 years. An accelerated elimination procedure could be used at any time after discontinuation of teriflunomide. Elimination can be accelerated by either of the following procedures:
- Administration of cholestyramine 8 g every 8 hours for 11 days. If cholestyramine 8 g three times a day is not well tolerated, cholestyramine 4 g three times a day can be used.
- Administration of 50 g oral activated charcoal powder every 12 hours for 11 days.
If either elimination procedure is poorly tolerated, treatment days do not need to be consecutive unless there is a need to lower teriflunomide plasma concentration rapidly.
At the end of 11 days, both regimens successfully accelerated teriflunomide elimination, leading to more than 98% decrease in teriflunomide plasma concentrations.
Use of the accelerated elimination procedure may potentially result in return of disease activity if the patient had been responding to teriflunomide treatment.
5.4 Bone Marrow Effects/Immunosuppression Potential/Infections
Bone Marrow Effects
A mean decrease compared to baseline in white blood cell (WBC) count of approximately 15% (mainly neutrophils and lymphocytes) and in platelet count of approximately 10% was observed in placebo-controlled trials in adult patients with 7 mg and 14 mg of teriflunomide. The decrease in mean WBC count occurred during the first 6 weeks and WBC count remained low during treatment. In placebo-controlled studies in adult patients, neutrophil count < 1.5x109/L was observed in 12% and 16% of patients receiving teriflunomide 7 mg and 14 mg, respectively, compared with 7% of patients receiving placebo; lymphocyte count <0.8x109/L was observed in 10% and 12% of patients receiving teriflunomide 7 mg and 14 mg, respectively, compared with 6% of patients receiving placebo. No cases of serious pancytopenia were reported in premarketing clinical trials of teriflunomide but rare cases of pancytopenia and agranulocytosis have been reported in the postmarketing setting with leflunomide. A similar risk would be expected for teriflunomide [see Clinical Pharmacology (12.3)]. Cases of thrombocytopenia with teriflunomide, including rare cases with platelet counts less than 50,000/mm3, have been reported in post-marketing setting. Obtain a complete blood cell count (CBC) within 6 months before the initiation of treatment with teriflunomide. Further monitoring should be based on signs and symptoms suggestive of bone marrow suppression.
Risk of Infection / Tuberculosis Screening
Patients with active acute or chronic infections should not start treatment until the infection(s) is resolved. If a patient develops a serious infection consider suspending treatment with teriflunomide and using an accelerated elimination procedure. Reassess the benefits and risks prior to resumption of therapy. Instruct patients receiving teriflunomide to report symptoms of infections to a physician.
Teriflunomide is not recommended for patients with severe immunodeficiency, bone marrow disease, or severe, uncontrolled infections. Medications like teriflunomide that have immunosuppression potential may cause patients to be more susceptible to infections, including opportunistic infections.
In placebo-controlled studies of teriflunomide in adult patients, no overall increase in the risk of serious infections was observed with teriflunomide 7 mg (2.2%) or 14 mg (2.7%) compared to placebo (2.2%).
However, one fatal case of klebsiella pneumonia sepsis occurred in a patient taking teriflunomide 14 mg for 1.7 years. Fatal infections have been reported in the postmarketing setting in patients receiving leflunomide, especially Pneumocystis jirovecii pneumonia and aspergillosis. Most of the reports were confounded by concomitant immunosuppressant therapy and/or comorbid illness which, in addition to rheumatoid disease, may predispose patients to infection. In clinical studies with teriflunomide, cytomegalovirus hepatitis reactivation has been observed.
In clinical studies with teriflunomide in adult patients, cases of tuberculosis have been observed. Prior to initiating teriflunomide, screen patients for latent tuberculosis infection with a tuberculin skin test or with a blood test for mycobacterium tuberculosis infection. Teriflunomide has not been studied in patients with a positive tuberculosis screen, and the safety of teriflunomide in individuals with latent tuberculosis infection is unknown. For patients testing positive in tuberculosis screening, treat by standard medical practice prior to therapy with teriflunomide.
Vaccination
No clinical data are available on the efficacy and safety of live vaccinations in patients taking teriflunomide. Vaccination with live vaccines is not recommended. The long half-life of teriflunomide should be considered when contemplating administration of a live vaccine after stopping teriflunomide.
Malignancy
The risk of malignancy, particularly lymphoproliferative disorders, is increased with the use of some immunosuppressive medications. There is a potential for immunosuppression with teriflunomide. No apparent increase in the incidence of malignancies and lymphoproliferative disorders was reported in the teriflunomide clinical trials, but larger and longer-term studies would be needed to determine whether there is an increased risk of malignancy or lymphoproliferative disorders with teriflunomide.
5.5 Hypersensitivity Reactions
Teriflunomide can cause anaphylaxis and severe allergic reactions [see Contraindications (4)]. Signs and symptoms have included dyspnea, urticaria, and angioedema including lips, eyes, throat, and tongue. Inform patients of the signs and symptoms of anaphylaxis and angioedema.
5.6 Serious Skin Reactions
Cases of serious skin reactions, sometimes fatal, including Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS) [see Warnings and Precautions (5.7)], have been reported with teriflunomide. Fatal outcomes were reported in one case of TEN and one case of DRESS. Inform patients of the signs and symptoms that may signal a serious skin reaction. Instruct patients to discontinue teriflunomide and seek immediate medical care should these signs and symptoms occur. Unless the reaction is clearly not drug related, discontinue teriflunomide and begin an accelerated elimination procedure immediately [see Warnings and Precautions (5.3)]. In such cases, patients should not be re-exposed to teriflunomide [see Contraindications (4)].
5.7 Drug Reaction with Eosinophilia and Systemic Symptoms
Drug reaction with eosinophilia and systemic symptoms (DRESS), also known as multiorgan hypersensitivity, has occurred with teriflunomide. One fatal case of DRESS that occurred in close temporal association (34 days) with the initiation of teriflunomide treatment has been reported in the postmarketing setting. DRESS typically, although not exclusively, presents with fever, rash, lymphadenopathy and/or facial swelling, in association with other organ system involvement, such as hepatitis, nephritis, hematologic abnormalities, myocarditis, or myositis, sometimes resembling an acute viral infection. Eosinophilia is often present. This disorder is variable in its expression, and other organ systems not noted here may be involved. It is important to note that early manifestations of hypersensitivity (e.g., fever, lymphadenopathy) may be present even though rash is not evident. If such signs or symptoms are present, the patient should be evaluated immediately. Discontinue teriflunomide, unless an alternative etiology for the signs or symptoms is established, and begin an accelerated elimination procedure immediately [see Warnings and Precautions (5.3)]. In such cases, patients should not be re-exposed to teriflunomide [see Contraindications (4)].
5.8 Peripheral Neuropathy
In placebo-controlled studies in adult patients, peripheral neuropathy, including both polyneuropathy and mononeuropathy (e.g., carpal tunnel syndrome), occurred more frequently in patients taking teriflunomide than in patients taking placebo. The incidence of peripheral neuropathy confirmed by nerve conduction studies was 1.4% (13 patients) and 1.9% (17 patients) of patients receiving 7 mg and 14 mg of teriflunomide, respectively, compared with 0.4% receiving placebo (4 patients). Treatment was discontinued in 0.7% (8 patients) with confirmed peripheral neuropathy (3 patients receiving teriflunomide 7 mg and 5 patients receiving teriflunomide 14 mg). Five of them recovered following treatment discontinuation. Not all cases of peripheral neuropathy resolved with continued treatment. Peripheral neuropathy also occurred in patients receiving leflunomide.
Age older than 60 years, concomitant neurotoxic medications, and diabetes may increase the risk for peripheral neuropathy. If a patient taking teriflunomide develops symptoms consistent with peripheral neuropathy, such as bilateral numbness or tingling of hands or feet, consider discontinuing teriflunomide therapy and performing an accelerated elimination procedure [see Warnings and Precautions (5.3)].
5.9 Increased Blood Pressure
In placebo-controlled studies in adult patients, the mean change from baseline to the end of study in systolic blood pressure was +2.3 mmHg and +2.7 mmHg for teriflunomide 7 mg and 14 mg, respectively, and -0.6 mmHg for placebo. The change from baseline in diastolic blood pressure was +1.4 mmHg and +1.9 mmHg for teriflunomide 7 mg and 14 mg, respectively, and -0.3 mmHg for placebo. Hypertension was an adverse reaction in 3.1% and 4.3% of patients treated with 7 mg or 14 mg of teriflunomide compared with 1.8% for placebo. Check blood pressure before start of teriflunomide treatment and periodically thereafter. Elevated blood pressure should be appropriately managed during treatment with teriflunomide.
5.10 Respiratory Effects
Interstitial lung disease, including acute interstitial pneumonitis, has been reported with teriflunomide in the postmarketing setting.
Interstitial lung disease and worsening of pre-existing interstitial lung disease have been reported during treatment with leflunomide. Interstitial lung disease may be fatal and may occur acutely at any time during therapy with a variable clinical presentation. New onset or worsening pulmonary symptoms, such as cough and dyspnea, with or without associated fever, may be a reason for discontinuation of therapy and for further investigation as appropriate. If discontinuation of the drug is necessary, consider initiation of an accelerated elimination procedure [see Warnings and Precautions (5.3)].
5.11 Pancreatitis in Pediatric Patients
Teriflunomide is not approved for use in pediatric patients. In the pediatric clinical trial, cases of pancreatitis were observed in 1.8% (2/109) of patients receiving teriflunomide; one of these cases was serious [see Use in Specific Populations (8.4)]. If pancreatitis is suspected, discontinue teriflunomide and start an accelerated elimination procedure [see Warnings and Precautions (5.3)].
5.12 Concomitant Use with Immunosuppressive or Immunomodulating Therapies
Coadministration with antineoplastic or immunosuppressive therapies used for treatment of multiple sclerosis has not been evaluated. Safety studies in which teriflunomide was concomitantly administered with other immune modulating therapies for up to one year (interferon beta, glatiramer acetate) did not reveal any specific safety concerns. The long term safety of these combinations in the treatment of multiple sclerosis has not been established. In any situation in which the decision is made to switch from teriflunomide to another agent with a known potential for hematologic suppression, it would be prudent to monitor for hematologic toxicity, because there will be overlap of systemic exposure to both compounds. Use of an accelerated elimination procedure may decrease this risk, but may also potentially result in return of disease activity if the patient had been responding to teriflunomide treatment [see Warnings and Precautions (5.3)].
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the prescribing information:
- Hepatotoxicity [see Contraindications (4) and Warnings and Precautions (5.1)]
- Bone Marrow Effects/Immunosuppression Potential/Infections [see Warnings and Precautions (5.4)]
- Hypersensitivity Reactions [see Contraindications (4) and Warnings and Precautions (5.5)]
- Serious Skin Reactions [see Warnings and Precautions (5.6)]
- Drug Reaction with Eosinophilia and Systemic Symptoms [see Warnings and Precautions (5.7)]
- Peripheral Neuropathy [see Warnings and Precautions (5.8)]
- Increased Blood Pressure [see Warnings and Precautions (5.9)]
- Respiratory Effects [see Warnings and Precautions (5.10)]
- Pancreatitis in Pediatric Patients [see Warnings and Precautions (5.11)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
A total of 2047 patients receiving teriflunomide (7 mg or 14 mg once daily) constituted the safety population in the pooled analysis of placebo controlled studies in patients with relapsing forms of multiple sclerosis; of these, 71% were female. The average age was 37 years.
Table 1 lists adverse reactions in placebo-controlled trials with rates that were at least 2% for teriflunomide patients and also at least 2% above the rate in placebo patients. The most common were headache, an increase in ALT, diarrhea, alopecia, and nausea. The adverse reaction most commonly associated with discontinuation was an increase in ALT (3.3%, 2.6%, and 2.3% of all patients in the teriflunomide 7 mg, teriflunomide 14 mg, and placebo treatment arms, respectively).
| Adverse Reaction | Teriflunomide 7 mg (N=1045) | Teriflunomide 14 mg (N=1002) | Placebo (N=997) |
|---|---|---|---|
| Headache | 18% | 16% | 15% |
| Increase in Alanine aminotransferase | 13% | 15% | 9% |
| Diarrhea | 13% | 14% | 8% |
| Alopecia | 10% | 13% | 5% |
| Nausea | 8% | 11% | 7% |
| Paresthesia | 8% | 9% | 7% |
| Arthralgia | 8% | 6% | 5% |
| Neutropenia | 4% | 6% | 2% |
| Hypertension | 3% | 4% | 2% |
Cardiovascular Deaths
Four cardiovascular deaths, including three sudden deaths, and one myocardial infarction in a patient with a history of hyperlipidemia and hypertension were reported among approximately 2600 patients exposed to teriflunomide in the premarketing database. These cardiovascular deaths occurred during uncontrolled extension studies, one to nine years after initiation of treatment. A relationship between teriflunomide and cardiovascular death has not been established.
Acute Renal Failure
In placebo-controlled studies, creatinine values increased more than 100% over baseline in 8/1045 (0.8%) patients in the 7 mg teriflunomide group and 6/1002 (0.6%) patients in the 14 mg teriflunomide group versus 4/997 (0.4%) patients in the placebo group. These elevations were transient. Some elevations were accompanied by hyperkalemia. Teriflunomide may cause acute uric acid nephropathy with transient acute renal failure because teriflunomide increases renal uric acid clearance.
Hypophosphatemia
In clinical trials, 18% of teriflunomide-treated patients had hypophosphatemia with serum phosphorus levels of at least 0.6 mmol/L, compared to 7% of placebo-treated patients; 4% of teriflunomide-treated patients had hypophosphatemia with serum phosphorus levels at least 0.3 mmol/L but less than 0.6 mmol/L, compared to 0.8% of placebo-treated patients. No patient in any treatment group had a serum phosphorus below 0.3 mmol/L.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of teriflunomide. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Blood and Lymphatic System Disorders: Thrombocytopenia [see Warnings and Precautions (5.4)]
- Gastrointestinal Disorders: Pancreatitis, colitis
- Hepatobiliary Disorders: Drug-induced liver injury (DILI) [see Warnings and Precautions (5.1)]
- Immune System Disorders: Hypersensitivity reactions, some of which were severe, such as anaphylaxis and angioedema [see Warnings and Precautions (5.5)]
- Respiratory, Thoracic, and Mediastinal Disorders: Interstitial lung disease [see Warnings and Precautions (5.10)]
- Skin and Subcutaneous Tissue Disorders: Severe skin reactions, including toxic epidermal necrolysis and Stevens-Johnson syndrome [see Warnings and Precautions (5.6)]; drug reaction with eosinophilia and systemic symptoms (DRESS) [see Warnings and Precautions (5.7)]; psoriasis or worsening of psoriasis (including pustular psoriasis and nail psoriasis); nail disorders
7 DRUG INTERACTIONS
Effect of Teriflunomide on CYP2C8 Substrates
Teriflunomide is an inhibitor of CYP2C8 in vivo. In patients taking teriflunomide, exposure of drugs metabolized by CYP2C8 (e.g., paclitaxel, pioglitazone, repaglinide, rosiglitazone) may be increased. Monitor these patients and adjust the dose of the concomitant drug(s) metabolized by CYP2C8 as required [see Clinical Pharmacology (12.3)].
Effect of Teriflunomide on Warfarin
Coadministration of teriflunomide with warfarin requires close monitoring of the international normalized ratio (INR) because teriflunomide may decrease peak INR by approximately 25%.
Effect of Teriflunomide on Oral Contraceptives
Teriflunomide may increase the systemic exposures of ethinylestradiol and levonorgestrel. Consideration should be given to the type or dose of contraceptives used in combination with teriflunomide [see Clinical Pharmacology (12.3)].
Effect of Teriflunomide on CYP1A2 Substrates
Teriflunomide may be a weak inducer of CYP1A2 in vivo. In patients taking teriflunomide, exposure of drugs metabolized by CYP1A2 (e.g., alosetron, duloxetine, theophylline, tizanidine) may be reduced. Monitor these patients and adjust the dose of the concomitant drug(s) metabolized by CYP1A2 as required[see Clinical Pharmacology (12.3)].
Effect of Teriflunomide on Organic Anion Transporter 3 (OAT3) Substrates
Teriflunomide inhibits the activity of OAT3 in vivo. In patients taking teriflunomide, exposure of drugs which are OAT3 substrates (e.g., cefaclor, cimetidine, ciprofloxacin, penicillin G, ketoprofen, furosemide, methotrexate, zidovudine) may be increased. Monitor these patients and adjust the dose of the concomitant drug(s) which are OAT3 substrates as required [see Clinical Pharmacology (12.3)].
Effect of Teriflunomide on BCRP and Organic Anion Transporting Polypeptide B1 and B3 (OATP1B1/1B3) Substrates
Teriflunomide inhibits the activity of BCRP and OATP1B1/1B3 invivo. For a patient taking teriflunomide, the dose of rosuvastatin should not exceed 10 mg once daily. For other substrates of BCRP (e.g., mitoxantrone) and drugs in the OATP family (e.g., methotrexate, rifampin), especially HMG-Co reductase inhibitors (e.g., atorvastatin, nateglinide, pravastatin, repaglinide, and simvastatin), consider reducing the dose of these drugs and monitor patients closely for signs and symptoms of increased exposures to the drugs while patients are taking teriflunomide [see Clinical Pharmacology (12.3)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Teriflunomide is contraindicated for use in pregnant women and females of reproductive potential not using effective contraception because of the potential for fetal harm based on animal data.[see Contraindications (4) and Warnings and Precautions (5.2)].
In animal reproduction studies in rat and rabbit, oral administration of teriflunomide during organogenesis caused teratogenicity and embryolethality at plasma exposures (AUC) lower than that at the maximum recommended human dose (MRHD) of 14 mg/day [see Data]. Available human data from pregnancy registries, clinical trials, pharmacovigilance cases, and published literature are too limited to draw any conclusions, but they do not clearly indicate increased birth defects or miscarriage associated with inadvertent teriflunomide exposure in the early first trimester when followed by an accelerated elimination procedure [see Clinical Considerations and Data]. There are no human data pertaining to exposures later in the first trimester or beyond.
In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively. The background risk of major birth defects and miscarriage in the indicated population is unknown.
Clinical Considerations
Fetal/Neonatal adverse reactions
Lowering the plasma concentration of teriflunomide by instituting an accelerated drug elimination procedure as soon as pregnancy is detected may decrease the risk to the fetus from teriflunomide. The accelerated drug elimination procedure includes verification that the plasma teriflunomide concentration is less than 0.02 mg/L [see Warnings and Precautions (5.3) and Clinical Pharmacology (12.3)].
Data
Human data
Available human data are limited. Prospectively reported data (from clinical trials and postmarketing reports) from >150 pregnancies in patients treated with teriflunomide and >300 pregnancies in patients treated with leflunomide have not demonstrated an increased rate of congenital malformations or miscarriage following teriflunomide exposure in the early first trimester when followed by an accelerated elimination procedure. Specific patterns of major congenital malformations in humans have not been observed. Limitations of these data include an inadequate number of reported pregnancies from which to draw conclusions, the short duration of drug exposure in reported pregnancies, which precludes a full evaluation of the fetal risks, incomplete reporting, and the inability to control for confounders (such as underlying maternal disease and use of concomitant medications).
Animal data
When teriflunomide (oral doses of 1, 3, or 10 mg/kg/day) was administered to pregnant rats throughout the period of organogenesis, high incidences of fetal malformation (primarily craniofacial, and axial and appendicular skeletal defects) and fetal death were observed at doses not associated with maternal toxicity. Adverse effects on fetal development were observed following dosing at various stages throughout organogenesis. Maternal plasma exposure at the no-effect level (1.0 mg/kg/day) for fetal developmental toxicity in rats was less than that in humans at the maximum recommended human dose (MRHD, 14 mg/day).
Administration of teriflunomide (oral doses of 1, 3.5, or 12 mg/kg/day) to pregnant rabbits throughout organogenesis resulted in high incidences of fetal malformation (primarily craniofacial, and axial and appendicular skeletal defects) and fetal death at doses associated with minimal maternal toxicity. Maternal plasma exposure at the no-effect dose (1.0 mg/kg/day) for fetal developmental toxicity in rabbits was less than that in humans at the MRHD.
In studies in which teriflunomide (oral doses of 0.05, 0.1, 0.3, 0.6, or 1.0 mg/kg/day) was administered to rats during gestation and lactation, decreased growth, eye and skin abnormalities, and high incidences of malformation (limb defects) and postnatal death were observed in the offspring at doses not associated with maternal toxicity. Maternal plasma exposure at the no-effect dose for prenatal and postnatal developmental toxicity in rats (0.10 mg/kg/day) was less than that in humans at the MRHD.
In animal reproduction studies of leflunomide, embryolethality and teratogenic effects were observed in pregnant rat and rabbit at or below clinically relevant plasma teriflunomide exposures (AUC). In published reproduction studies in pregnant mice, leflunomide was embryolethal and increased the incidence of malformations (craniofacial, axial skeletal, heart and great vessel). Supplementation with exogenous uridine reduced the teratogenic effects in pregnant mice, suggesting that the mode of action (inhibition of mitochondrial enzyme dihydroorotate dehydrogenase) is the same for therapeutic efficacy and developmental toxicity. At recommended doses in humans, teriflunomide and leflunomide result in a similar range of plasma concentrations of teriflunomide.
8.2 Lactation
Risk Summary
There are no data on the presence of teriflunomide in human milk, the effects on the breastfed infant, or the effects on milk production. Teriflunomide was detected in rat milk following a single oral dose. Because of the potential for adverse reactions in a breastfed infant from teriflunomide, women should not breastfeed during treatment with teriflunomide.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Exclude pregnancy prior to initiation of treatment with teriflunomide in females of reproductive potential. Advise females to notify their healthcare provider immediately if pregnancy occurs or is suspected during treatment [see Warnings and Precautions (5.2, 5.3) and Use in Specific Populations (8.1)].
Contraception
Females
Females of reproductive potential should use effective contraception while taking teriflunomide. If teriflunomide is discontinued, use of contraception should be continued until it is verified that plasma concentrations of teriflunomide are less than 0.02 mg/L (0.02 mcg/mL, the level expected to have minimal fetal risk, based on animal data).
Females of reproductive potential who wish to become pregnant should discontinue teriflunomide and undergo an accelerated elimination procedure. Effective contraception should be used until it is verified that plasma concentrations of teriflunomide are less than 0.02 mg/L (0.02 mcg/mL) [see Warnings and Precautions (5.2, 5.3) and Use in Specific Population (8.1)].
Males
Teriflunomide is detected in human semen. Animal studies to specifically evaluate the risk of male mediated fetal toxicity have not been conducted. To minimize any possible risk, men not wishing to father a child and their female partners should use effective contraception. Men wishing to father a child should discontinue use of teriflunomide and either undergo an accelerated elimination procedure or wait until verification that the plasma teriflunomide concentration is less than 0.02 mg/L (0.02 mcg/mL) [see Warnings and Precautions (5.3)].
8.4 Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Effectiveness of teriflunomide for the treatment of relapsing form of multiple sclerosis in pediatric patients (10 to 17 years of age) was not established in an adequate and well-controlled clinical study in 166 patients (109 patients received once daily doses of teriflunomide and 57 patients received placebo) for up to 96 weeks. Pancreatitis has been reported in adults in the postmarketing setting, but appears to occur at higher frequency in the pediatric population. In this pediatric study, cases of pancreatitis were reported in 1.8% (2/109) of patients who received teriflunomide compared to no patients in the placebo group. All patients in the pediatric trial recovered or were recovering after treatment discontinuation and accelerated elimination procedure [see Warnings and Precautions (5.11)].
Additionally, elevated or abnormal blood creatine phosphokinase was reported in 6.4% of pediatric patients who received teriflunomide compared to no patients in the placebo group.
Juvenile Animal Toxicity Data
Oral administration of teriflunomide (0, 0.3, 3, or 6 mg/kg/day) to young rats on postnatal days 21 to 70 resulted in suppression of immune function (T-cell dependent antibody response) at the mid and high doses, and adverse effects on male reproductive organs (reduced sperm count) and altered neurobehavioral function (increased locomotor activity) at the high dose. At the no-effect dose (0.3 mg/kg/day) for developmental toxicity in juvenile rats, plasma exposures were less than those in pediatric patients at the doses of teriflunomide tested in the clinical study.
8.6 Hepatic Impairment
No dosage adjustment is necessary for patients with mild and moderate hepatic impairment. The pharmacokinetics of teriflunomide in severe hepatic impairment has not been evaluated. Teriflunomide is contraindicated in patients with severe hepatic impairment [see Contraindications (4) Warnings and Precautions (5.1), and Clinical Pharmacology (12.3)].
8.7 Renal Impairment
No dosage adjustment is necessary for patients with mild, moderate, and severe renal impairment [see Clinical Pharmacology (12.3)].
10 OVERDOSAGE
There is no experience regarding teriflunomide overdose or intoxication in humans. Teriflunomide 70 mg daily up to 14 days was well tolerated by healthy subjects.
In the event of clinically significant overdose or toxicity, cholestyramine or activated charcoal is recommended to accelerate elimination [see Warnings and Precautions (5.3)].
11 DESCRIPTION
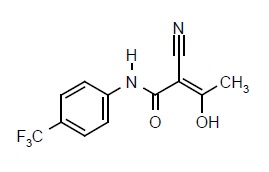
Teriflunomide is an oral de novo pyrimidine synthesis inhibitor of the DHO-DH enzyme, with the chemical name (Z)-2-Cyano-3-hydroxy-but-2-enoic acid-(4-trifluoromethylphenyl)-amide. Its molecular weight is 270.21, and the empirical formula is C12H9F3N2O2 with the following chemical structure:
Teriflunomide is a white to off-white solid that is practically insoluble in acetone, slightly soluble in ethanol, very slightly soluble in polyethylene glycol and isopropanol, and insoluble in water.
Teriflunomide is formulated as film-coated tablets for oral administration. Teriflunomide tablet contain 7 mg or 14 mg of teriflunomide and the following inactive ingredients: colloidal silicon dioxide, maize starch B, hydroxypropyl cellulose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and sodium starch glycolate. The film coating for the 7 mg and 14 mg tablet contains hypromellose, titanium dioxide, polyethylene glycol and talc.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Teriflunomide, an immunomodulatory agent with anti-inflammatory properties, inhibits dihydroorotate dehydrogenase, a mitochondrial enzyme involved in de novo pyrimidine synthesis. The exact mechanism by which teriflunomide exerts its therapeutic effect in multiple sclerosis is unknown but may involve a reduction in the number of activated lymphocytes in CNS.
12.2 Pharmacodynamics
Potential to Prolong the QT Interval
In a placebo-controlled thorough QT study performed in healthy adult subjects, there was no evidence that teriflunomide caused QT interval prolongation of clinical significance (i.e., the upper bound of the 90% confidence interval for the largest placebo-adjusted, baseline-corrected QTc was below 10 ms).
12.3 Pharmacokinetics
Teriflunomide is the principal active metabolite of leflunomide and is responsible for leflunomide's activity in vivo. At recommended doses, teriflunomide and leflunomide result in a similar range of plasma concentrations of teriflunomide.
Based on a population analysis of teriflunomide in healthy adult volunteers and adult MS patients, median t1/2 was approximately 18 and 19 days after repeated doses of 7 mg and 14 mg respectively. It takes approximately 3 months respectively to reach steady-state concentrations. The estimated AUC accumulation ratio is approximately 30 after repeated doses of 7 or 14 mg.
Absorption
Median time to reach maximum plasma concentrations is between 1 to 4 hours post dose following oral administration of teriflunomide.
Food does not have a clinically relevant effect on teriflunomide pharmacokinetics.
Distribution
Teriflunomide is extensively bound to plasma protein (>99%) and is mainly distributed in plasma. The volume of distribution is 11 L after a single intravenous (IV) administration.
Metabolism
Teriflunomide is the major circulating moiety detected in plasma. The primary biotransformation pathway to minor metabolites of teriflunomide is hydrolysis, with oxidation being a minor pathway. Secondary pathways involve oxidation, N-acetylation and sulfate conjugation.
Elimination
Teriflunomide is eliminated mainly through direct biliary excretion of unchanged drug as well as renal excretion of metabolites. Over 21 days, 60.1% of the administered dose is excreted via feces (37.5%) and urine (22.6%). After an accelerated elimination procedure with cholestyramine, an additional 23.1% was recovered (mostly in feces). After a single IV administration, the total body clearance of teriflunomide is 30.5 mL/h.
Drug Interaction Studies
Teriflunomide is not metabolized by Cytochrome P450 or flavin monoamine oxidase enzymes.
The potential effect of teriflunomide on other drugs
-
CYP2C8 substrates
There was an increase in mean repaglinide Cmax and AUC (1.7- and 2.4-fold, respectively), following repeated doses of teriflunomide and a single dose of 0.25 mg repaglinide, suggesting that teriflunomide is an inhibitor of CYP2C8 in vivo. The magnitude of interaction could be higher at the recommended repaglinide dose [see Drug Interactions (7)].
-
CYP1A2 substrates
Repeated doses of teriflunomide decreased mean Cmax and AUC of caffeine by 18% and 55%, respectively, suggesting that teriflunomide may be a weak inducer of CYP1A2 in vivo [see Drug Interactions (7)].
-
OAT3 substrates
There was an increase in mean cefaclor Cmax and AUC (1.43- and 1.54-fold, respectively), following repeated doses of teriflunomide, suggesting that teriflunomide is an inhibitor of organic anion transporter 3 (OAT3) in vivo [see Drug Interactions (7)].
-
BCRP and OATP1B1/1B3 substrates
There was an increase in mean rosuvastatin Cmax and AUC (2.65- and 2.51-fold, respectively), following repeated doses of teriflunomide, suggesting that teriflunomide is an inhibitor of BCRP transporter and organic anion transporting polypeptide 1B1 and 1B3 (OATP1B1/1B3) [see Drug Interactions (7)].
-
Oral contraceptives
There was an increase in mean ethinylestradiol Cmax and AUC0–24 (1.58- and 1.54-fold, respectively) and levonorgestrel Cmax and AUC0–24 (1.33- and 1.41-fold, respectively) following repeated doses of teriflunomide [see Drug Interactions (7)].
- Teriflunomide did not affect the pharmacokinetics of bupropion (a CYP2B6 substrate), midazolam (a CYP3A4 substrate), S-warfarin (a CYP2C9 substrate), omeprazole (a CYP2C19 substrate), and metoprolol (a CYP2D6 substrate).
Specific Populations
-
Hepatic impairment
Mild and moderate hepatic impairment had no impact on the pharmacokinetics of teriflunomide. The pharmacokinetics of teriflunomide in severe hepatic impairment has not been evaluated [see Contraindications (4), Warnings and Precautions (5.1), and Use in Specific Populations (8.6)].
-
Renal impairment
Severe renal impairment had no impact on the pharmacokinetics of teriflunomide [see Use in Specific Populations (8.7)].
- Gender
In a population analysis, the clearance rate for teriflunomide is 23% less in females than in males.
- Race
Effect of race on the pharmacokinetics of teriflunomide cannot be adequately assessed due to a low number of non-white patients in the clinical trials.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
No evidence of carcinogenicity was observed in lifetime carcinogenicity bioassays in mouse and rat. In mouse, teriflunomide was administered orally at doses up to 12 mg/kg/day for up to 95 to 104 weeks; plasma teriflunomide exposures (AUC) at the highest dose tested are approximately 3 times that in humans at the maximum recommended human dose (MRHD, 14 mg/day). In rat, teriflunomide was administered orally at doses up to 4 mg/kg/day for up to 97 to 104 weeks; plasma teriflunomide AUCs at the highest doses tested are less than that in humans at the MRHD.
Mutagenesis
Teriflunomide was negative in the in vitro bacterial reverse mutation (Ames) assay, the in vitro HPRT assay, and in in vivo micronucleus and chromosomal aberration assays. Teriflunomide was positive in an in vitro chromosomal aberration assay in human lymphocytes, with and without metabolic activation. Addition of uridine (to supplement the pyrimidine pool) reduced the magnitude of the clastogenic effect; however, teriflunomide was positive in the in vitro chromosomal aberration assay, even in the presence of uridine.
4-Trifluoromethylaniline (4-TFMA), a minor metabolite of teriflunomide, was positive in the in vitro bacterial reverse mutation (Ames) assay, the in vitro HPRT assay, and the in vitro chromosomal aberration assay in mammalian cells. 4-TFMA was negative in in vivo micronucleus and chromosomal aberration assays.
Impairment of Fertility
Oral administration of teriflunomide (0, 1, 3, 10 mg/kg/day) to male rats prior to and during mating (to untreated females) resulted in no adverse effects on fertility; however, reduced epididymal sperm count was observed at the mid and high doses tested. The no-effect dose for reproductive toxicity in male rats (1 mg/kg) is less than the MRHD on a mg/m2 basis.
Oral administration of teriflunomide (0, 0.84, 2.6, 8.6 mg/kg/day) to female rats, prior to and during mating (to untreated males) and continuing to gestation day 6, resulted in embryolethality, reduced fetal body weight, and/or malformations at all doses tested. Due to marked embryolethality at the highest dose tested, no fetuses were available for evaluation. The lowest dose tested is less than the MRHD on a mg/m2 basis.
14 CLINICAL STUDIES
Four randomized, controlled, double-blind clinical trials established the efficacy of teriflunomide in patients with relapsing forms of multiple sclerosis.
Study 1 was a double-blind, placebo-controlled clinical trial that evaluated once daily doses of teriflunomide 7 mg and teriflunomide 14 mg for up to 26 months in patients with relapsing forms of multiple sclerosis. Patients were required to have a diagnosis of multiple sclerosis exhibiting a relapsing clinical course, with or without progression, and to have experienced at least one relapse over the year preceding the trial or at least two relapses over the two years preceding the trial. Patients were required not to have received interferon-beta for at least four months, or any other multiple sclerosis medication for at least six months before entering the study, nor were these medications permitted during the study. Neurological evaluations were to be performed at screening, every 12 weeks until week 108, and after suspected relapses. MRI was to be performed at screening, and at week 24, 48, 72, and 108. The primary endpoint was the annualized relapse rate (ARR).
In Study 1, 1088 patients were randomized to receive teriflunomide 7 mg (n=366), teriflunomide 14 mg (n=359), or placebo (n=363). At entry, patients had an Expanded Disability Status Scale (EDSS) score ≤5.5. Patients had a mean age of 38 years, mean disease duration of 5 years, and mean EDSS at baseline of 2.7. A total of 91% of patients had relapsing remitting multiple sclerosis, and 9% had a progressive form of multiple sclerosis with relapses. The mean duration of treatment was 635, 627, and 631 days for teriflunomide 7 mg, teriflunomide 14 mg, and placebo, respectively. The percentage of patients who completed the study treatment period was 75%, 73%, and 71% for teriflunomide 7 mg, teriflunomide 14 mg, and placebo, respectively.
There was a statistically significant reduction in ARR for patients who received teriflunomide 7 mg or teriflunomide 14 mg, compared to patients who received placebo (see Table 2). There was a consistent reduction of the ARR noted in subgroups defined by sex, age group, prior multiple sclerosis therapy, and baseline disease activity.
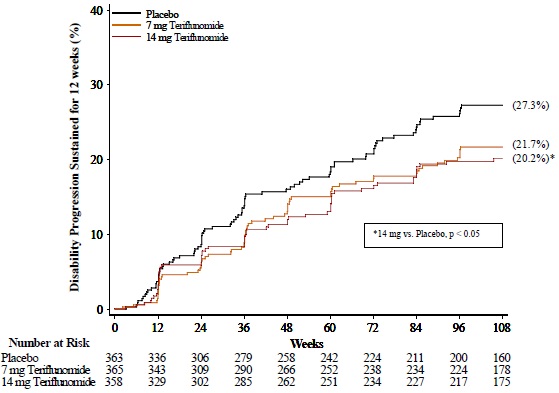
There was a statistically significant reduction in the relative risk of disability progression at week 108 sustained for 12 weeks (as measured by at least a 1-point increase from baseline EDSS ≤ 5.5 or a 0.5 point increase for those with a baseline EDSS > 5.5) in the teriflunomide 14 mg group compared to placebo (see Table 2 and Figure 1).
The effect of teriflunomide on several magnetic resonance imaging (MRI) variables including the total lesion volume of T2 and hypointense T1 lesions, was assessed in Study 1. The change in total lesion volume from baseline was significantly lower in the teriflunomide 7 mg and teriflunomide 14 mg groups than in the placebo group. Patients in both teriflunomide groups had significantly fewer gadolinium-enhancing lesions per T1-weighted scan than those in the placebo group (see Table 2).
| Teriflunomide 7 mg N=365 | Teriflunomide 14 mg N=358 | Placebo N=363 |
|
|---|---|---|---|
| Clinical Endpoints | |||
| Annualized relapse rate | 0.370 (p = 0.0002) | 0.369 (p = 0.0005) | 0.539 |
| Relative risk reduction | 31% | 31% | |
| Percent of patients remaining relapse-free at week 108 | 53.7% | 56.5% | 45.6% |
| Percent disability progression at week 108 | 21.7% (p = 0.084) | 20.2% (p = 0.028) | 27.3% |
| Hazard ratio | 0.76 | 0.70 | |
| MRI Endpoints | |||
| Median change from baseline in Total lesion volume* (mL) at week 108 | 0.755 (p= 0.0317 )† | 0.345 (p = 0.0003 )† | 1.127 |
| Mean number of Gd-enhancing T1-lesions per scan | 0.570 (p < 0.0001) | 0.261 (p < 0.0001) | 1.331 |
| Figure 1: Kaplan-Meier Plot of Time to Disability Progression Sustained for 12 Weeks (Study 1) |
|
|
Study 2 was a double-blind, placebo-controlled clinical trial that evaluated once daily doses of teriflunomide 7 mg and teriflunomide 14 mg for up to 40 months in patients with relapsing forms of multiple sclerosis. Patients were required to have a diagnosis of multiple sclerosis exhibiting a relapsing clinical course and to have experienced at least one relapse over the year preceding the trial, or at least two relapses over the two years preceding the trial. Patients were required not to have received any multiple sclerosis medication for at least three months before entering the trial, nor were these medications permitted during the trial. Neurological evaluations were to be performed at screening, every 12 weeks until completion, and after every suspected relapse. The primary end point was the ARR.
A total of 1165 patients received teriflunomide 7 mg (n=407), teriflunomide 14 mg (n=370), or placebo (n=388). Patients had a mean age of 38 years, a mean disease duration of 5 years, and a mean EDSS at baseline of 2.7. A total of 98% of patients had relapsing remitting multiple sclerosis, and 2% had a progressive form of multiple sclerosis with relapses. The mean duration of treatment was 552, 567, and 571 days for teriflunomide 7 mg, teriflunomide 14 mg, and placebo, respectively. The percentage of patients who completed the study treatment period was 67%, 66%, and 68% for teriflunomide 7 mg, teriflunomide 14 mg, and placebo, respectively.
There was a statistically significant reduction in the ARR for patients who received teriflunomide 7 mg or teriflunomide 14 mg compared to patients who received placebo (see Table 3). There was a consistent reduction of the ARR noted in subgroups defined by sex, age group, prior multiple sclerosis therapy, and baseline disease activity.
There was a statistically significant reduction in the relative risk of disability progression at week 108 sustained for 12 weeks (as measured by at least a 1-point increase from baseline EDSS ≤ 5.5 or a 0.5 point increase for those with a baseline EDSS > 5.5) in the teriflunomide 14 mg group compared to placebo (see Table 3 and Figure 2).
| Teriflunomide 7 mg N=407 | Teriflunomide 14 mg N=370 | Placebo N=388 |
|
|---|---|---|---|
| Clinical Endpoints | |||
| Annualized relapse rate | 0.389 (p = 0.0183) | 0.319 (p = 0.0001) | 0.501 |
| Relative risk reduction | 22% | 36% | |
| Percent of patients remaining relapse- free at week 108 | 58.2% | 57.1% | 46.8% |
| Percent disability progression at week 108 | 21.1% (p = 0.762) | 15.8% (p = 0.044) | 19.7% |
| Hazard ratio | 0.96 | 0.69 |
| Figure 2: Kaplan-Meier Plot of Time to Disability Progression Sustained for 12 Weeks (Study 2) |
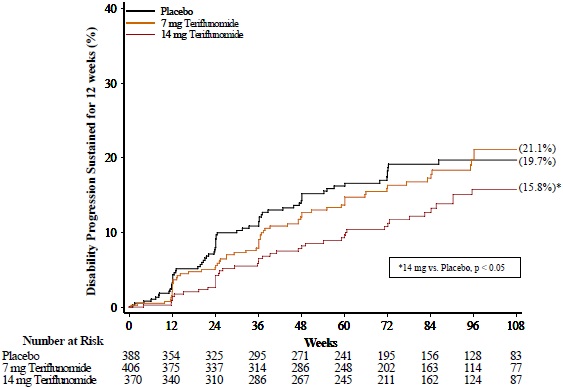 |
Study 3 was a double-blind, placebo-controlled clinical trial that evaluated once daily doses of teriflunomide 7 mg and teriflunomide 14 mg for up to 108 weeks in patients with relapsing multiple sclerosis. Patients were required to have had a first clinical event consistent with acute demyelination occurring within 90 days of randomization with 2 or more T2 lesions at least 3 mm in diameter that were characteristic of multiple sclerosis. A total of 614 patients received teriflunomide 7 mg (n=203), teriflunomide 14 mg (n=214), or placebo (n=197). Patients had a mean age of 32 years, EDSS at baseline of 1.7, and mean disease duration of two months. The proportion of patients free of relapse was greater in the teriflunomide 7 mg (70.5%, p <0.05) and teriflunomide 14 mg (72.2%, p <0.05) groups than in the placebo group (61.7%).
The effect of teriflunomide on MRI activity was also demonstrated in Study 4, a randomized, double-blind, placebo-controlled clinical trial of multiple sclerosis patients with relapse. In Study 4, MRI was to be performed at baseline, 6 weeks, 12 weeks, 18 weeks, 24 weeks, 30 weeks, and 36 weeks after treatment initiation. A total of 179 patients were randomized to teriflunomide 7 mg (n=61), teriflunomide 14 mg (n=57), or placebo (n= 61). Baseline demographics were consistent across treatment groups. The primary endpoint was the average number of unique active lesions/MRI scan during treatment. The mean number of unique active lesions per brain MRI scan during the 36-week treatment period was lower in patients treated with teriflunomide 7 mg (1.06) and teriflunomide 14 mg (0.98) as compared to placebo (2.69), the difference being statistically significant for both (p=0.0234 and p=0.0052, respectively).
16 HOW SUPPLIED/STORAGE AND HANDLING
Teriflunomide is available as 7 mg and 14 mg tablets.
The 14 mg tablet is white to off white, pentagonal, film coated tablet debossed with B on one side and 14 on the other side. Each tablet contains 14 mg of teriflunomide.
The 7 mg tablet is white to off white, hexagonal, film coated tablet debossed with B on one side and 7 on the other side. Each tablet contains 7 mg of teriflunomide.
Teriflunomide tablet 14 mg are supplied as:
NDC 70377-018-91 Carton of 28 tablets containing 1 wallet composed of 2 folded blister cards of 14 tablets per blister card
NDC 70377-018-11 Bottle of 30 tablets
Teriflunomide tablet 7 mg are supplied as:
NDC 70377-017-91 Carton of 28 tablets containing 1 wallet composed of 2 folded blister cards of 14 tablets per blister card
NDC 70377-017-11 Bottle of 30 tablets
Store at 68°F to 77°F (20°C to 25°C). Excursions permitted between 59°F and 86°F (15°C and 30°C).
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
A Medication Guide is required for distribution with teriflunomide tablets.
Hepatotoxicity
Inform patients that teriflunomide may cause liver injury, which can be life-threatening, and that their liver enzymes will be checked before starting teriflunomide and at least monthly for 6 months after starting teriflunomide [see Dosage and Administration (2) and Warnings and Precautions (5.1)]. Advise patients that they should contact their physician if they have any unexplained nausea, vomiting, abdominal pain, fatigue, anorexia, or jaundice and/or dark urine.
Embryofetal Toxicity
- Advise females of reproductive potential
- of the potential for fetal harm if teriflunomide is taken during pregnancy
- to notify their healthcare provider immediately if a pregnancy occurs or is suspected
- to use effective contraception during treatment with teriflunomide and until the teriflunomide plasma concentration is verified to be less than 0.02 mg/L [see Warnings and Precautions (5.2, 5.3), Use in Specific Populations (8.1, 8.3), Clinical Pharmacology (12.3)]
- Instruct men taking teriflunomide and not wishing to father a child to use effective contraception to minimize any possible risk to the fetus; their female partners should also use effective contraception.
- Advise men wishing to father a child to discontinue use of teriflunomide and undergo an accelerated elimination procedure.
Availability of an Accelerated Elimination Procedure
Advise patients that teriflunomide may stay in the blood for up to 2 years after the last dose and that an accelerated elimination procedure may be used if needed [see Warnings and Precautions (5.3)].
Risk of Infections
Inform patients that they may develop a lowering of their white blood cell counts and that their blood counts will be checked before starting teriflunomide.
Inform patients that they may be more likely to get infections when taking teriflunomide and that they should contact their physician if they develop symptoms of infection, particularly in case of fever [see Warnings and Precautions (5.4)].
Advise patients that the use of some vaccines should be avoided during treatment with teriflunomide and for at least 6 months after discontinuation.
Hypersensitivity Reactions
Advise patients to discontinue teriflunomide and seek immediate medical attention if any signs or symptoms of a hypersensitivity reaction occur [see Contraindications (4) and Warnings and Precautions (5.5)]. Signs and symptoms can include dyspnea, urticaria, angioedema involving the lips, eyes, throat, or tongue, or skin rash.
Serious Skin Reactions
Advise patients to discontinue teriflunomide and seek immediate medical attention if any signs of a serious skin reaction, such as SJS or TEN, occur [see Warnings and Precautions (5.6)]. Signs and symptoms can include rash, mouth sores, blisters, or peeling skin.
DRESS/Multi-organ Hypersensitivity
Instruct patients and caregivers that a fever or rash associated with signs of other organ system involvement (e.g., lymphadenopathy, hepatic dysfunction) may be drug-related and should be reported to their healthcare provider immediately. Teriflunomide should be discontinued immediately if a serious hypersensitivity reaction is suspected [see Warnings and Precautions (5.7)].
Peripheral Neuropathy
Inform patients that they may develop peripheral neuropathy. Advise patients that they should contact their physician if they develop symptoms of peripheral neuropathy, such as numbness or tingling of hands or feet [see Warnings and Precautions (5.8)].
Increased Blood Pressure
Inform patients that teriflunomide may increase blood pressure [see Warnings and Precautions (5.9)].
Lactation
Advise females not to breastfeed during treatment with teriflunomide [see Use in Specific Populations (8.2)].
Manufactured by:
Biocon Pharma Limited
Bengaluru,
India - 560099
Manufactured for:
Biocon Pharma Inc.,
Iselin, New Jersey, 08830-3009
United States of America
Revised: 01/2023
Medication Guide
Teriflunomide (ter" i floo' noe mide) tablet, for oral use
Read this Medication Guide before you start using teriflunomide tablet and each time you get a refill. There may be new information. This information does not take the place of talking with your doctor about your medical condition or your treatment.
What is the most important information I should know about teriflunomide tablet?
Teriflunomide tablet may cause serious side effects, including:
-
Liver problems: Teriflunomide tablet may cause serious liver problems, including liver failure that can be life-threatning and may require a liver transplant. Your risk of developing serious liver problems may be higher if you already have liver problems or take other medicines that also affect your liver. Your doctor should do blood tests to check your liver:
- within 6 months before you start taking teriflunomide tablet
- 1 time a month for 6 months after you start taking teriflunomide tablet
- nausea
- vomiting
- stomach pain
- loss of appetite
- tiredness
- your skin or the whites of your eyes turn yellow
- dark urine
-
Harm to your unborn baby: Teriflunomide tablet may cause harm to your unborn baby. Do not take teriflunomide tablet if you are pregnant. Do not take teriflunomide tablet unless you are using effective birth control.
- If you are a female, you should have a pregnancy test before you start taking teriflunomide tablet. Use effective birth control during your treatment with teriflunomide tablet.
- After stopping teriflunomide tablet, continue using effective birth control until you have blood tests to make sure your blood levels of teriflunomide are low enough. If you become pregnant while taking teriflunomide tablet or within 2 years after you stop taking it, tell your doctor right away.
-
For men taking teriflunomide tablet:
- If your female partner plans to become pregnant, you should stop taking teriflunomide tablet and ask your doctor how to quickly lower the levels of teriflunomide in your blood.
- If your female partner does not plan to become pregnant, you and your female partner should use effective birth control during your treatment with teriflunomide tablet. Teriflunomide remains in your blood after you stop taking it, so continue using effective birth control until teriflunomide blood levels have been checked and they are low enough.
Teriflunomide may stay in your blood for up to 2 years after you stop taking it. Your doctor can prescribe a medicine to help lower your blood levels of teriflunomide more quickly. Talk to your doctor if you want more information about this.
What is teriflunomide tablet?
Teriflunomide tablet is a prescription medicine used to treat relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.
It is not known if teriflunomide tablets are safe and effective in children.
Who should not take teriflunomide tablet?
Do not take teriflunomide tablet if you:
- have severe liver problems
- are pregnant or are of childbearing age and not using effective birth control
- have had an allergic reaction to leflunomide, teriflunomide, or any other ingredients in teriflunomide tablet. Please see the end of this Medication Guide for a complete list of ingredients in teriflunomide tablet.
- take a medicine called leflunomide
What should I tell my doctor before taking teriflunomide tablet?
Before you take teriflunomide tablet, tell your doctor about all of your medical conditions, including if you:
- have liver or kidney problems
- have a fever or infection, or you are unable to fight infections
- have numbness or tingling in your hands or feet that is different from your MS symptoms
- have diabetes
- have had serious skin problems when taking other medicines
- have breathing problems
- have high blood pressure
- are breastfeeding or plan to breastfeed. It is not known if teriflunomide passes into your breast milk. You and your doctor should decide if you will take teriflunomide tablet or breastfeed. You should not do both.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Using teriflunomide tablet and other medicines may affect each other causing serious side effects. Teriflunomide tablet may affect the way other medicines work, and other medicines may affect how teriflunomide tablet work.
Especially tell your doctor if you take medicines that could raise your chance of getting infections, including medicines used to treat cancer or to control your immune system.
Ask your doctor or pharmacist for a list of these medicines if you are not sure.
Know the medicines you take. Keep a list of them to show your doctor or pharmacist when you get a new medicine.
How should I take teriflunomide tablet?
- Take teriflunomide tablet exactly as your doctor tells you to take it.
- Take teriflunomide tablet 1 time each day.
- Take teriflunomide tablet with or without food.
What are possible side effects of teriflunomide tablet?
Teriflunomide tablet may cause serious side effects, including:
- see " What is the most important information I should know about teriflunomide tablet? "
-
decreases in your white blood cell count. Your white blood cell counts should be checked before you start taking teriflunomide tablet. When you have a low white blood cell count you:
-
may have more frequent infections. You should have a skin test for TB (tuberculosis) before you start taking teriflunomide tablet. Tell your doctor if you have any of these symptoms of an infection:
- fever
- tiredness
- body aches
- chills
- nausea
- vomiting
-
should not receive certain vaccinations during your treatment with teriflunomide tablet and for 6 months after your treatment with teriflunomide tablet ends.
•allergic reactions. Stop taking teriflunomide tablet and call your doctor right away or get emergency medical help if you have difficulty breathing, itching, swelling on any part of your body including in your lips, eyes, throat, or tongue.
•serious skin reactions. Teriflunomide tablet may cause serious skin reactions that may lead to death. Stop taking teriflunomide tablet and call your doctor right away or get emergency medical help if you have any of the following symptoms: rash or redness and peeling, mouth sores or blisters.
•other types of allergic reactions or serious problems that may affect different parts of the body such as your liver, kidneys, heart, or blood cells. You may or may not have a rash with these types of reactions. Other symptoms you may have are:
-
severe muscle pain
-
swollen lymph glands
-
swelling of your face
-
unusual bruising or bleeding
-
weakness or tiredness
-
yellowing of your skin or the white part of your eyes
If you have a fever or rash with any of the above symptoms, stop taking teriflunomide tablet and call your doctor right away
-
may have more frequent infections. You should have a skin test for TB (tuberculosis) before you start taking teriflunomide tablet. Tell your doctor if you have any of these symptoms of an infection:
-
numbness or tingling in your hands or feet that is different from your MS symptoms. You have a higher chance of getting these symptoms if you:
- are over 60 years of age
- take certain medicines that affect your nervous system
- have diabetes
- high blood pressure. Your doctor should check your blood pressure before you start taking teriflunomide tablet and while you are taking teriflunomide tablet.
-
new or worsening breathing problems. These may be serious and lead to death. Call your doctor right away or get emergency medical help if you have shortness of breath or coughing with or without fever
The most common side effects of teriflunomide tablet include:
- headache
- diarrhea
- nausea
- hair thinning or loss (alopecia)
- increases in the results of blood tests to check your liver function
These are not all the possible side effects of teriflunomide tablet. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store teriflunomide tablet?
- Store teriflunomide tablet at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep teriflunomide tablet and all medicines out of the reach of children.
General information about the safe and effective use of teriflunomide tablet.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use teriflunomide tablet for a condition for which it was not prescribed. Do not give teriflunomide tablet to other people, even if they have the same symptoms you have. It may harm them.
You can ask your doctor or pharmacist for information about teriflunomide tablets that is written for health professionals.
What are the ingredients in teriflunomide tablet?
Active ingredient: teriflunomide
Inactive ingredients in 7 mg and 14 mg tablets: colloidal silicon dioxide, maize starch B, hydroxypropyl cellulose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and sodium starch glycolate. The film coating for the 14 mg and 7 mg tablet contains hypromellose, polyethylene glycol, talc and titanium dioxide.
For more information, call Biocon Pharma Inc., at 1-866-924-6266
Manufactured by:
Biocon Pharma Limited
Bengaluru,
India - 560099
Manufactured for:
Biocon Pharma Inc.,
Iselin, New Jersey, 08830-3009
United States of America
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Revised: January 2023