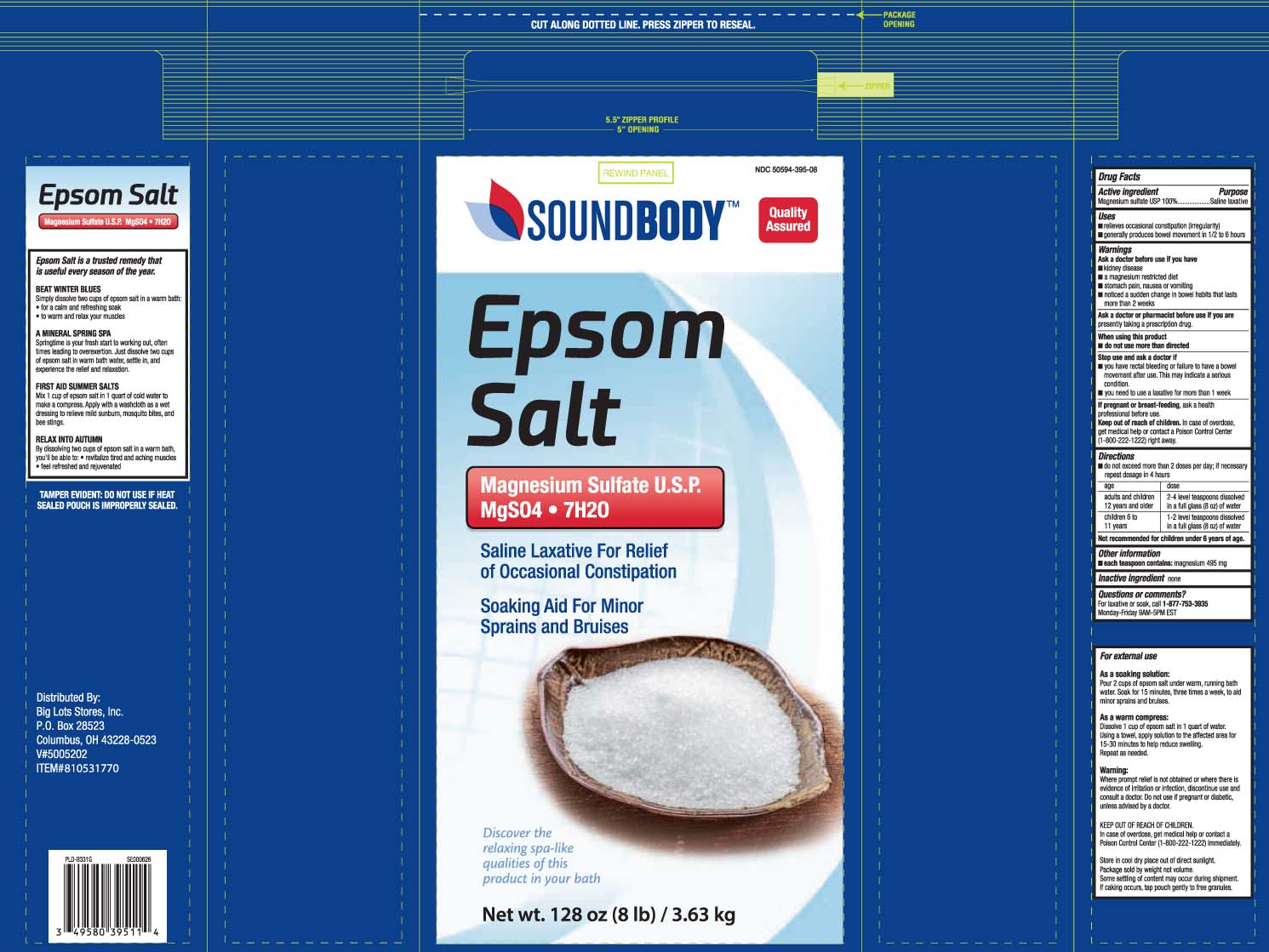
Uses
- relieves occasional constipation (irregularity)
- generally produces bowel movement in 1/2 to 6 hours
Warnings
Ask a doctor before use if you have
- kidney disease
- a magnesium restricted diet
- stomach pain, nausea or vomiting
- noticed a sudden change in bowel habits that lasts more than 2 weeks
Directions
- do not exceed more than 2 doses per day; if necessary repeat dosage in 4 hours
| age | dose |
| adults and children 12 years and older | 2-4 level teaspoons dissolved in a full glass (8oz) of water |
| children 6 to 11 years |
1-2 level teaspoons dissolved in a full glass (8oz) of water |
Not recommended for children under 6 years of age.
Principal Display Panel
EPSOM SALTS
Magnesium Sulfate U.S.P.
Saline Laxative For Relief of Occasional Constipation
Soaking Aid For Minor Sprains and Bruises
Net Wt oz LB (kg)
TAMPER EVIDENT: DO NOT USE IF HEAT SEALED POUCH IS IMPROPERLY SEALED
Distributed By:
Big Lots Stores, Inc.
P.O. Box 28523
Columbus, OH 43228-0523