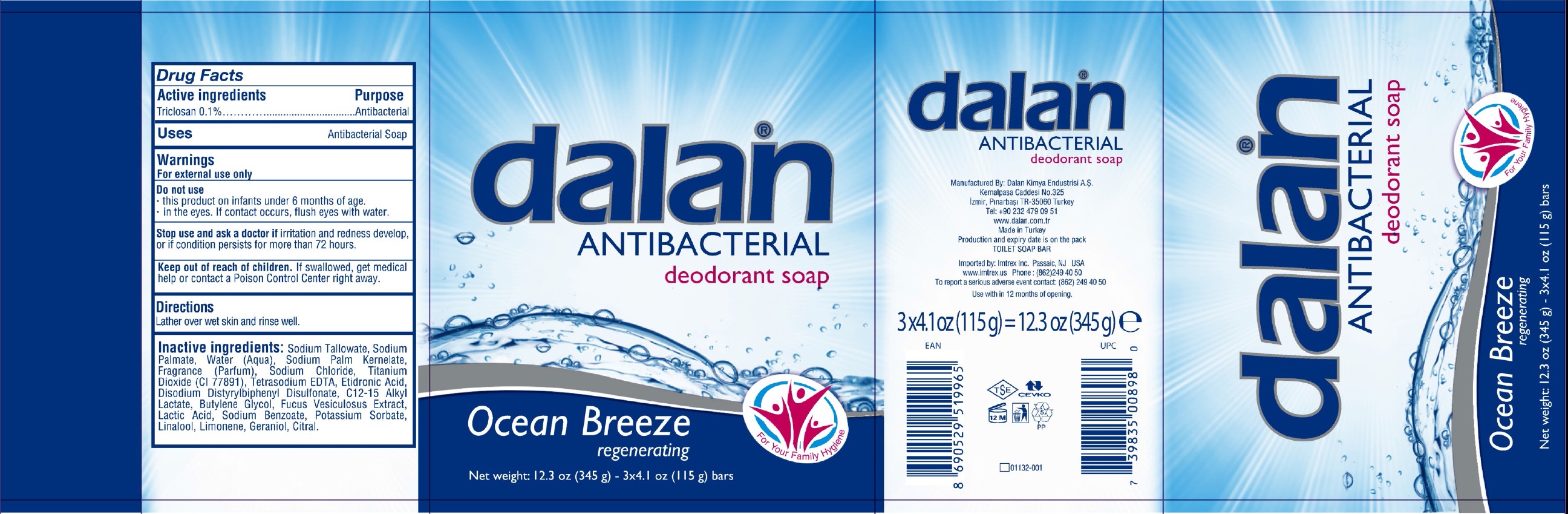
DALAN ANTIBACTERIAL DEODORANT OCEAN BREEZE- triclosan soap
Dalan Kimya Endustri A.S.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredients
Triclosan 0.1%
Purpose
Antibacterial Soap
Warnings
For external use only
Do not use
- this product on infants under 6 months of age.
- in the eyes. If contact occurs, flush eyes with water.
Stop use and ask a doctor if
irritation and redness develop, or if condition persists for more than 72 hours.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Lather over wet skin and rinse well.
Inactive ingredients:
Sodium Tallowate, Sodium Palmate, Water (Aqua), Sodium Palm Kernelate, Fragrance (Parfum), Sodium Chloride, Titanium Dioxide (CI 77891), Tetrasodium EDTA, Etidronic Acid, Disodium Distyrylbiphenyl Disulfonate, C12-15 Alkyl Lactate, Butylene Glycol, Fucus Vesiculosus Extract, Lactice Acid, Sodium Benzoate, Potassium Sorbate, Linalool, Limonene, Geraniol, Citral.
Package Labeling

Dalan Kimya Endustri A.S.

