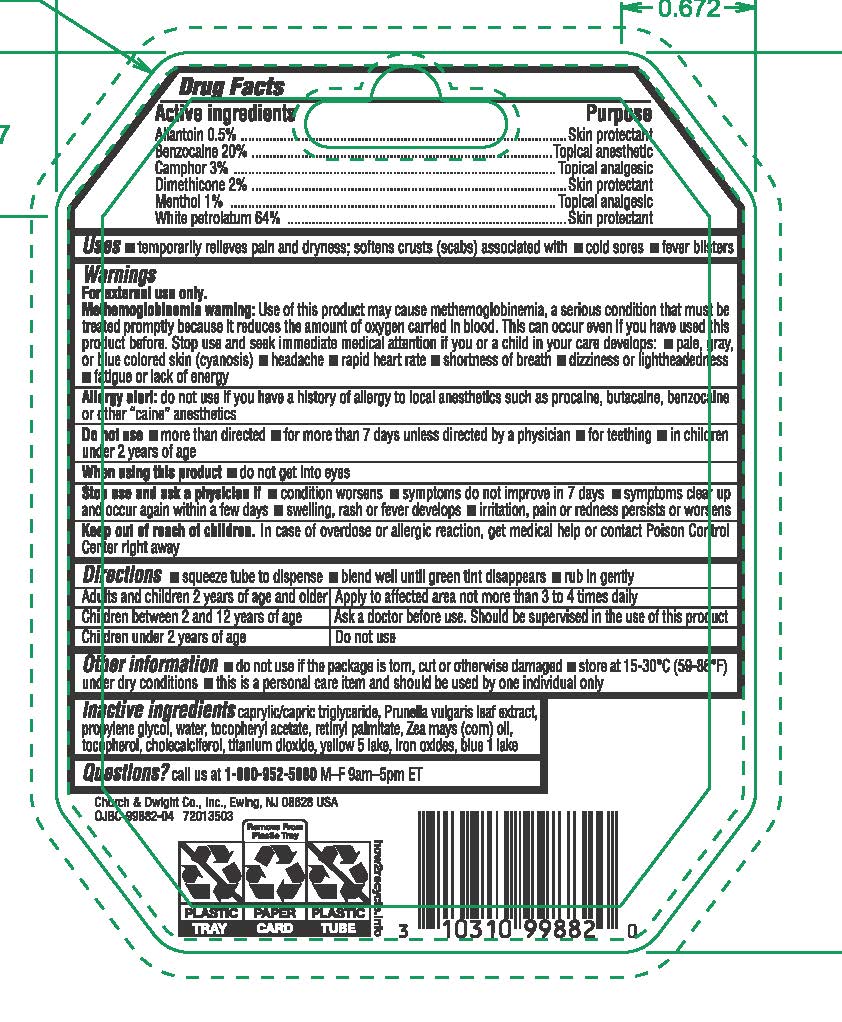
Allantoin - Skin protectant
Benzocaine - Topical anesthetic
Camphor - Topical analgesic
Dimethicone - Skin protectant
Menthol - Topical analgesic
White petrolatum - Skin protectant
Uses • temporarily relieves pain and dryness; softens crusts (scabs) associated with • cold sores • fever blisters
For external use only.
Allergy alert: do not use if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other "caine" anesthetics
Stop use and ask a physician if • conditions worsens • symptoms do not improve in 7 days • symptoms clear up and occur again within a few days • swelling, rash or fever develops • irritation, pain or redness persists or worsens
Keep out of reach of children. In case of overdose or allergic reaction, get medical help or contact Poison Control Center right away.
Directions
• squeeze tube to dispense • blend well until green tint disappears • rub in gently
Adults and children 2 years of age and older | Apply to affected area not more than 3 to 4 times daily
Children under 12 years of age | Should be supervised in the use of this product
Children under 2 years of age | Ask a physician
Other information • do not use if the package is torn, cut or otherwise damaged • store at 15-30ºC (59-86º) under dry conditions • this is a personal care item and should be used by one individual only
Inactive Ingredients caprylic/capric triglyceride, Prunella vulgaris leaf extract. propylene glycol, water, tocopheryl acetate, retinyl palmitate, Zea mays (corn) oil, tocopherol, cholecalciferol, titanium dioxide, yellow 5 lake, iron oxides, blue 1 lake