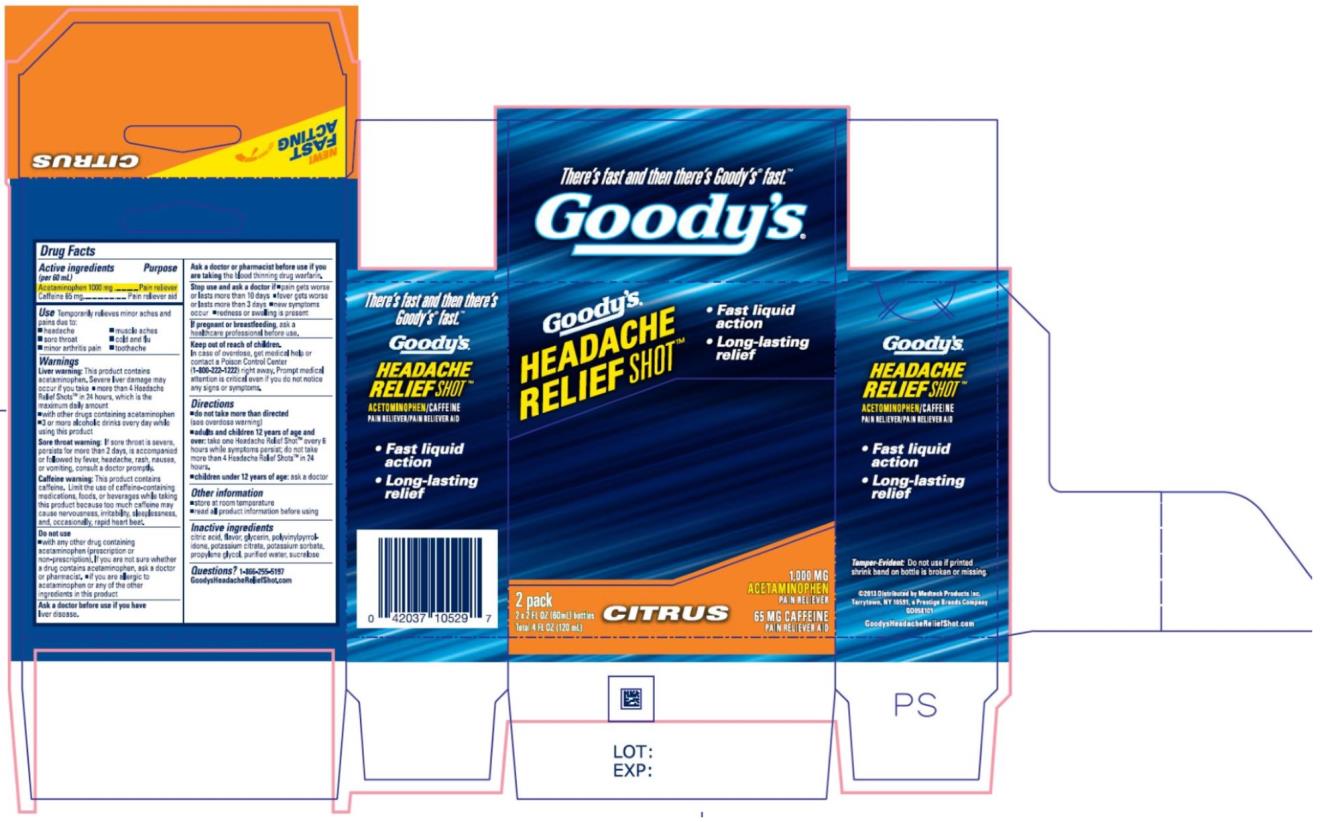
Use
Temporarily relieves minor aches and pains due to:
• headache
• sore throat
• minor arthritis pain
• muscle aches
• cold and flu
• toothache
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if
you take
• more than 4 Headache Relief Shots™ in 24 hours, which is the maximum daily amount
• with other drugs containing acetaminophen
• 3 or more alcoholic drinks every day while using this product
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Caffeine warning: This product contains caffeine. Limit the use of caffeine-containing medications, foods, or beverages while taking this product because too much caffeine may cause nervousness, irritability, sleeplessness, and, occasionally, rapid heart beat.
Do not use
• with any other drug containing acetaminophen (prescription or non-prescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
• if you are allergic to acetaminophen or any of the inactive ingredients in this product
Directions
• do not take more than directed (see overdose warning)
• adults and children 12 years of age and over: take one Headache Relief Shot every 6 hours while symptoms persist; do not take more than 4 Headache Relief Shots in 24 hours.
• children under 12 years of age: ask a doctor