SUPPLEMENT FACTS
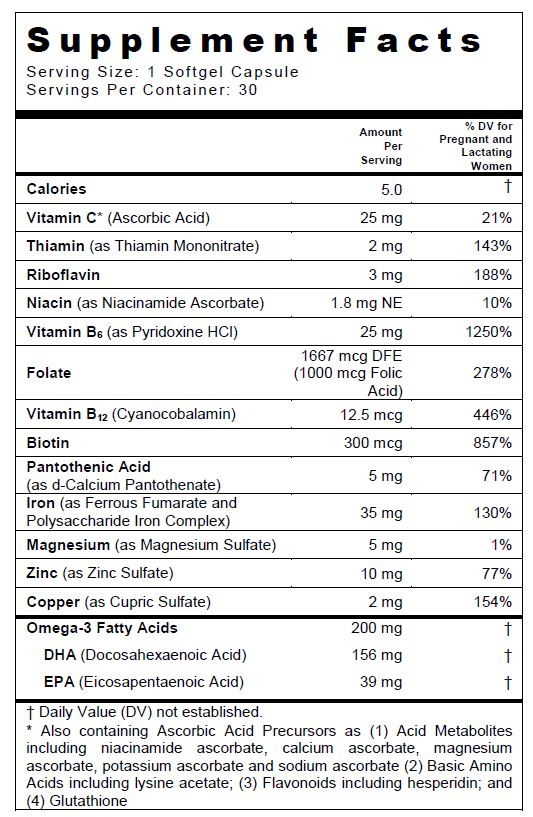
Other Ingredients: Bovine Gelatin, Glycerin, Yellow Beeswax, Soy Lecithin, Purified Water, Orange Cream Flavor O.S, Sorbitol, Titanium Dioxide, Ethyl Vanillin, FD&C Blue #1.
THIS PRODUCT CONTAINS SOY AND FISH (TUNA, MAY INCLUDE ANCHOVY AND/OR SARDINE).
Taron™-C DHA is a prescription multivitamin/mineral indicated to provide omega-3 fatty acid supplementation throughout pregnancy, during the postnatal period for both lactating and non-lactating mothers, and throughout the childbearing years. Taron™-C DHA may be useful in improving the nutritional status of women prior to conception.
CONTRAINDICATIONS
Taron™-C DHA is contraindicated in patients with a known hypersensitivity to any of the ingredients including soy or fish products. All iron compounds are contraindicated in patients with hemochromatosis, hemosiderosis, or hemolytic anemias.
WARNINGS
Administration of omega-3 fatty acids should be avoided in patients taking anticoagulants and in those known to have an inherited or acquired predisposition to bleeding.
| WARNING:Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. KEEP THIS PRODUCT OUT OF REACH OF CHILDREN. In case of accidental overdose, call a doctor or poison control center immediately. |
PRECAUTIONS
Folic acid alone is an improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. Folic acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurologic manifestations remain progressive.
The patient’s medical conditions and consumption of other drugs, herbs, and/or supplements should be considered.
Pediatric Use: Safety and effectiveness in pediatric populations have not been established.
Geriatric Use: Safety and effectiveness in elderly populations have not been established.
Biotin levels higher than the recommended daily allowance may cause interference with some laboratory tests, including cardiovascular diagnostic tests (e.g. troponin) and hormone tests, and may lead to incorrect test results. Tell your healthcare provider about all prescription and over-the-counter medicines, vitamins, and dietary supplements that you take, including biotin.
DRUG INTERACTIONS
Taron™-C DHA is not recommended for and should not be given to patients receiving levodopa because the action of levodopa is antagonized by pyridoxine. There is a possibility of increased bleeding due to pyridoxine interaction with anticoagulants (e.g., Aspirin, Heparin, Clopidogrel).
ADVERSE REACTIONS
Folic Acid: Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
Ferrous Fumarate: Gastrointestinal disturbances (anorexia, nausea, diarrhea, constipation) occur occasionally, but are usually mild and may subside with continuation of therapy. Although the absorption of iron is best when taken between meals, giving Taron™-C DHA after meals may control occasional G.I. disturbances. Taron™-C DHA is best absorbed when taken at bedtime.
OVERDOSAGE
Iron is toxic. Acute overdosage of iron may cause nausea and vomiting and, in severe cases, cardiovascular collapse and death. Other symptoms include pallor and cyanosis, melena, shock, drowsiness, and coma. The estimated overdose of orally ingested iron is 300-mg/kg body weight. When overdoses are ingested by children, severe reactions, including fatalities, have resulted. Taron™-C DHA should be stored beyond the reach of children to prevent against accidental iron poisoning.
Treatment: For specific therapy, exchange transfusion and chelating agents should be used. For general management, perform gastric lavage with sodium bicarbonate solution or milk. Administer intravenous fluids and electrolytes and use oxygen.
DESCRIPTION
Taron™-C DHA is a blue, oval softgel capsule containing a dark brown oil, imprinted with “T536”.
DIRECTIONS FOR USE
Adults (over 12 years of age) may take one capsule daily between meals or as prescribed by a physician. Do not exceed recommended dosage. Do not administer to children under the age of 12.
HOW SUPPLIED
Taron™-C DHA is dispensed in child-resistant bottles of 30 softgel capsules.
PRODUCT CODE 13811-536-30
STORAGE
Store at 20°-25°C (68°-77°F); excursions permitted to 15°-30°C (59°- 86°F). [See USP Controlled Room Temperature].
KEEP OUT OF REACH OF CHILDREN
For use on the order of a healthcare practitioner.
Call your doctor about side effects. To report side effects, call Trigen Laboratories, LLC at 1-770-509-4500 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Rev. 12/2021
PLR-TARONCDHA-00001-2
Manufactured for:
Trigen Laboratories, LLC
Alpharetta, GA 30005
www.trigenlab.com

