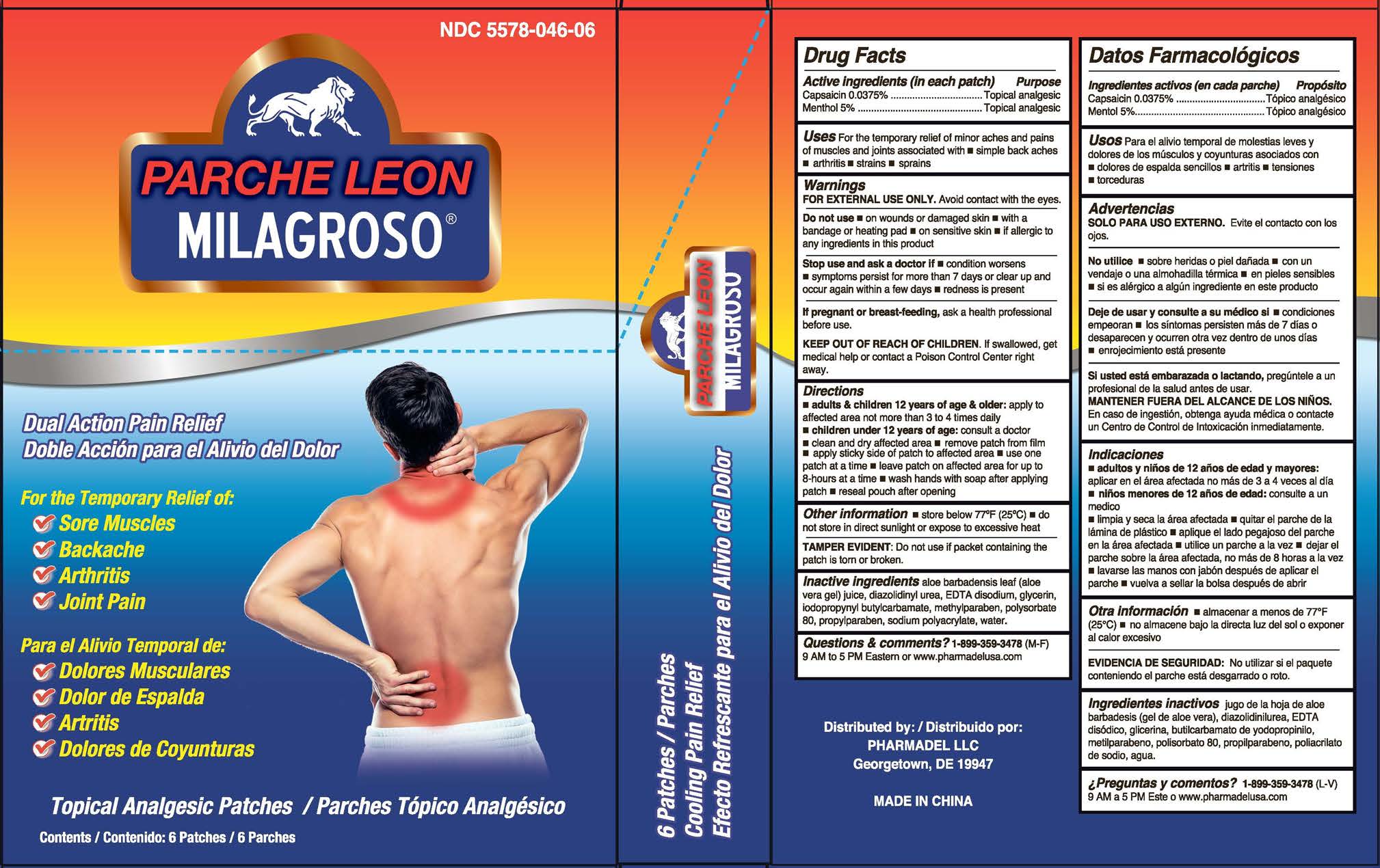
Uses
For the temporary relief of minor aches and pains of muscles and joints associated with
- simple backaches
- arthritis
- strains
- sprains
Warnings
FOR EXTERNAL USE ONLY. Avoid contact with the eyes.
Allergy Alert: This product contains natural rubber latex which may cause allergy reactions.
Do not use
- on wounds or damaged skin
- with a bandage or heating pad
- on sensitive skin
- if allergic to any ingredients in this product
KEEP OUT OF REACH OF CHILDREN.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- adults & children 12 yrs of age & older: apply to affected area not more than 3 to 4 times daily
- children under 2 year of age: consult a doctor
- clean and dry affected area
- remove patch from film
- apply sticky side of patch to affected area
- use one patch at a time
- leave patch on affected area for up to 8-hours at a time
Inactive ingredients:
aloe barbadensis leaf (aloe vera gel) juice, diazolidinyl urea, EDTA disodium, glycerin, iodopropynyl butylcarbamate, methylparaben, polysorbate 80, propylparaben, sodium polyacrylate, water.